principles of pharmacology: pharmacokinetics: lecture 2/3
1/89
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
90 Terms
pharmacodynamics
drug actions and their mechanisms
pharmacokinetics
drug movement into, within, and out of the body
what are the four key points of pharmacokinetics
absorption, distribution, metabolism, excretion (clearance)
what are the mechanisms of drug transport
passive diffusion, filtration, carrier mediated transport (active transport and facilitated diffusion), and endocytosis
passive diffusion
low molecular weight drugs that are both water and lipid soluble dissolve in membrane and cross to the other side. primary means by which drugs cross membranes
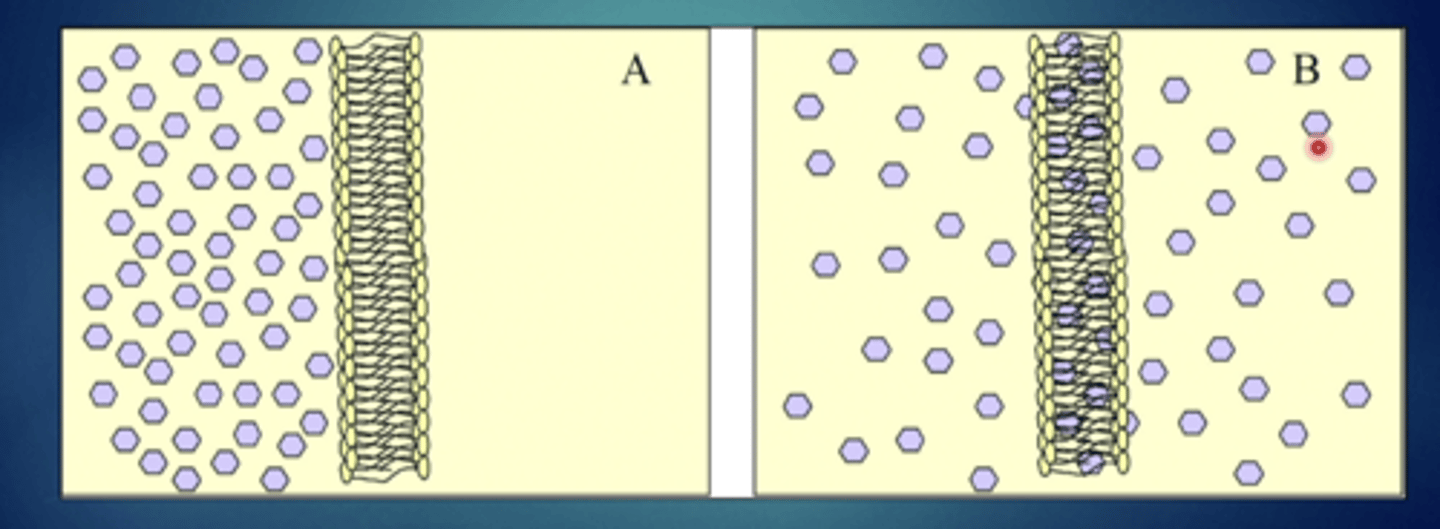
explain this image - passive diffusion (non-electrolytes)
substances will move from high concentration to low concentration and then reach equilibrium so they will be in equal concentrations in both compartments, rapid increase gradual decrease over time
electrolytes
substances that can be ionized, if they are ionize they can not cross the membrane, membranes repel ionized molecules
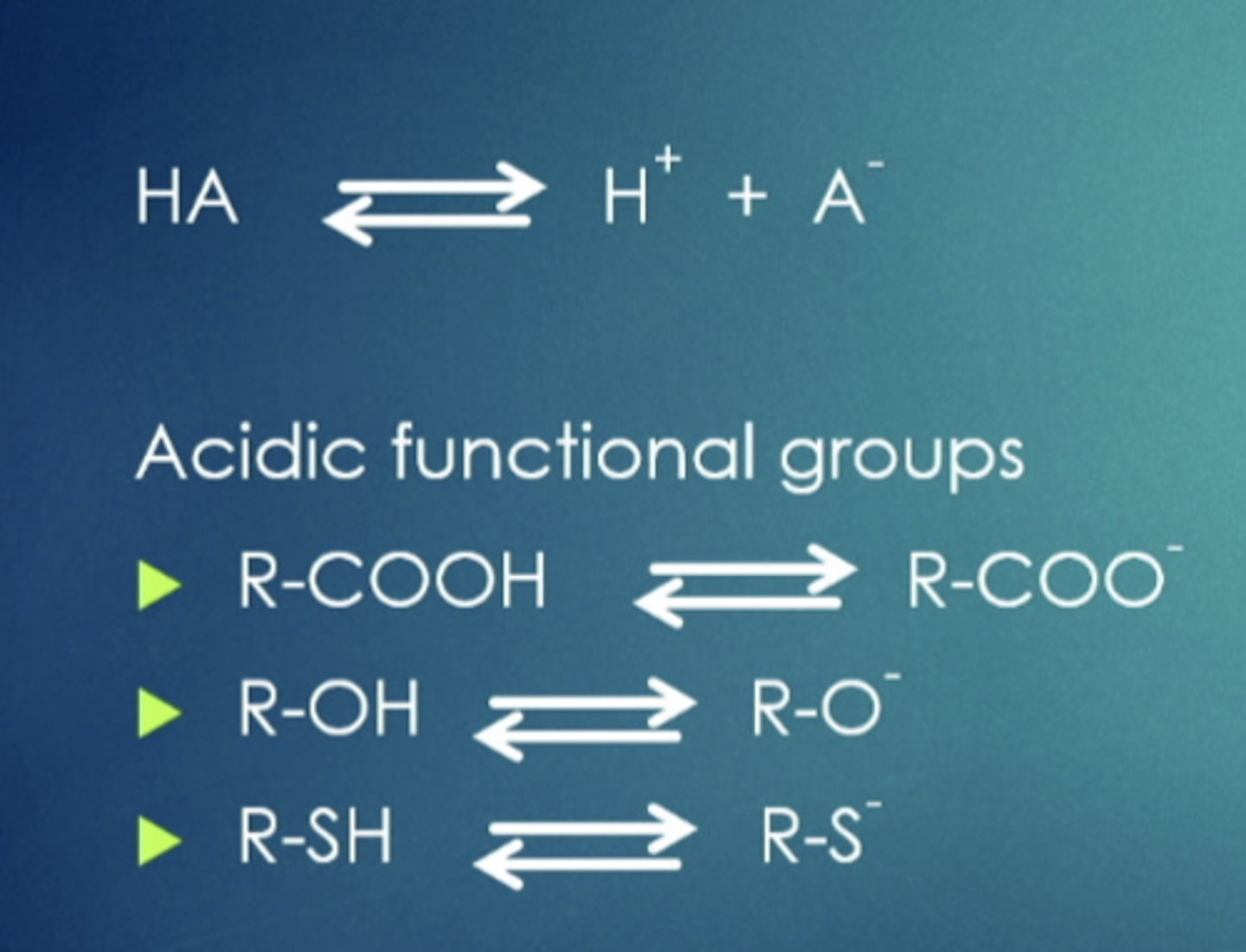
weak acids
can cross in certain pH but can also be repelled in different ones
weak bases
same as weak acids, can cross in certain pH but can also be repelled in different ones
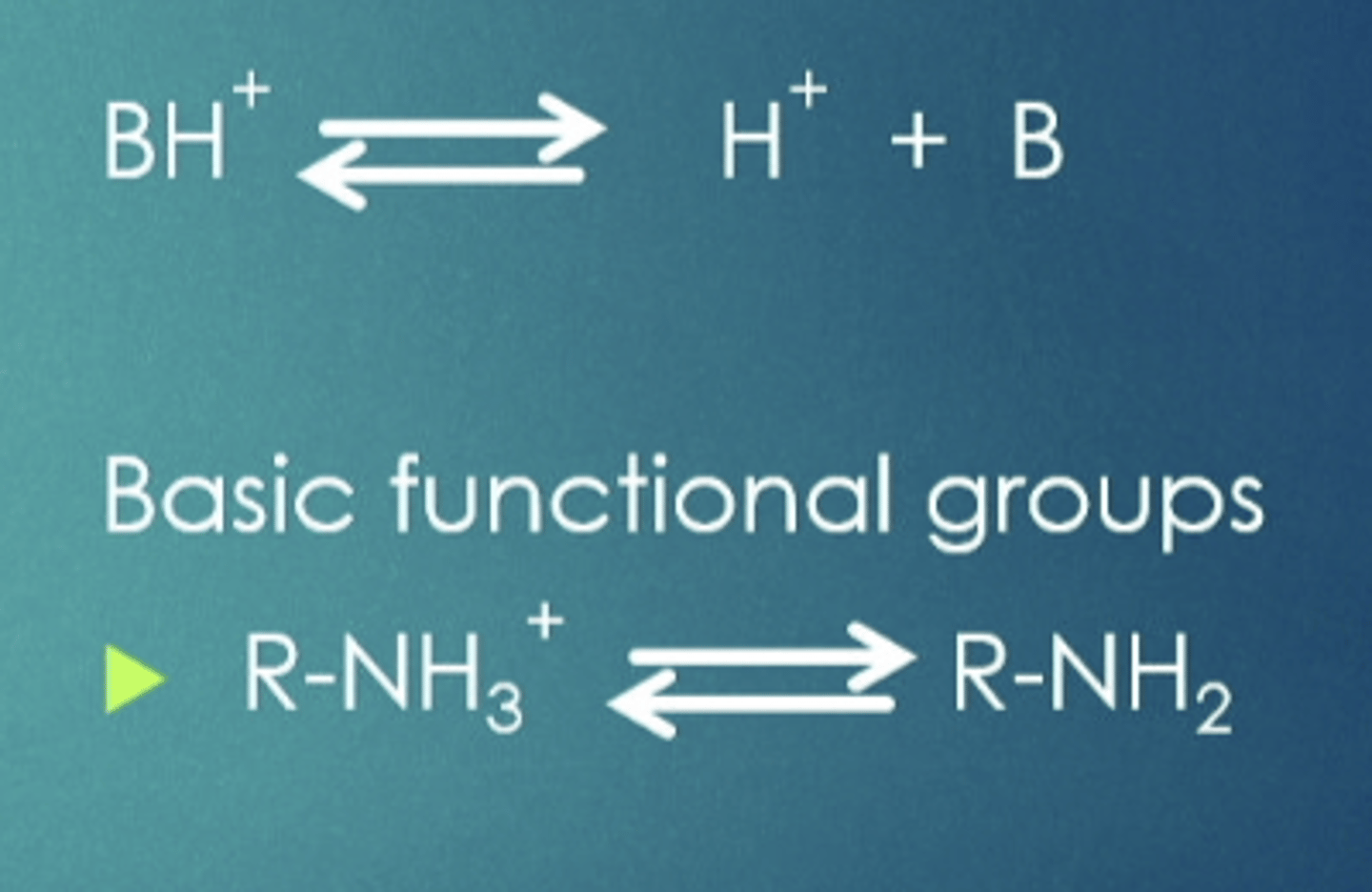
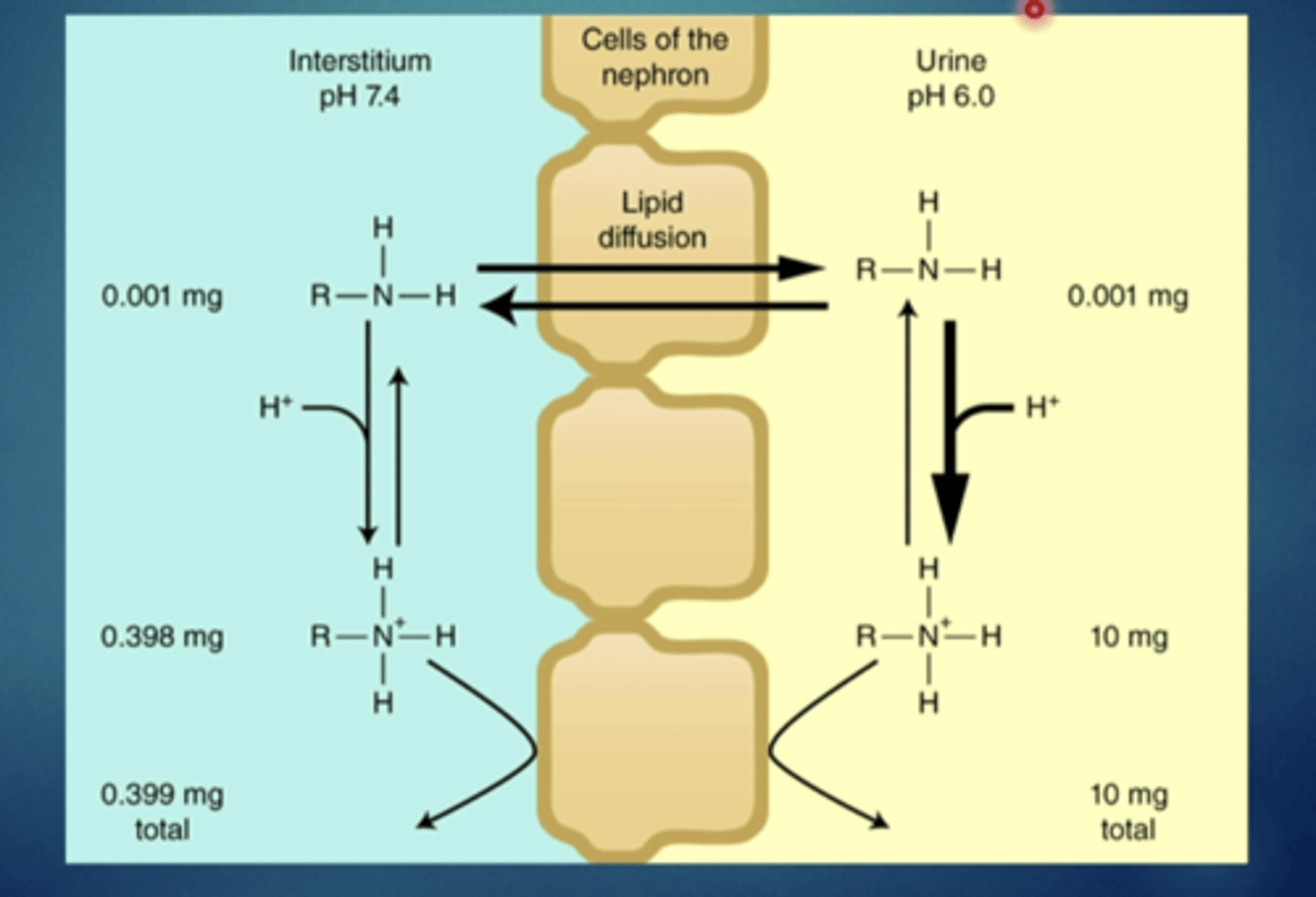
explain this image - passive diffusion (electrolytes)
if there is a difference in ph across different barriers then you will see a concentrating or trapping effect on the other side
a weak base will be trapped on the...
more acidic side (lower ph) because weak bases are more ionized in lower ph environments
a weak acid will be trapped on the ...
more basic side (higher ph)
if a drug gets trapped it will become ...
less effective
if there is an infection the area becomes ..
highly acidic so a local anesthetic becomes less effective
influence of ph on ionizable drugs
many drugs are weak acids or weak bases, present in solution as both lipid-soluble, non-ionized form and lipid insoluble ionized form
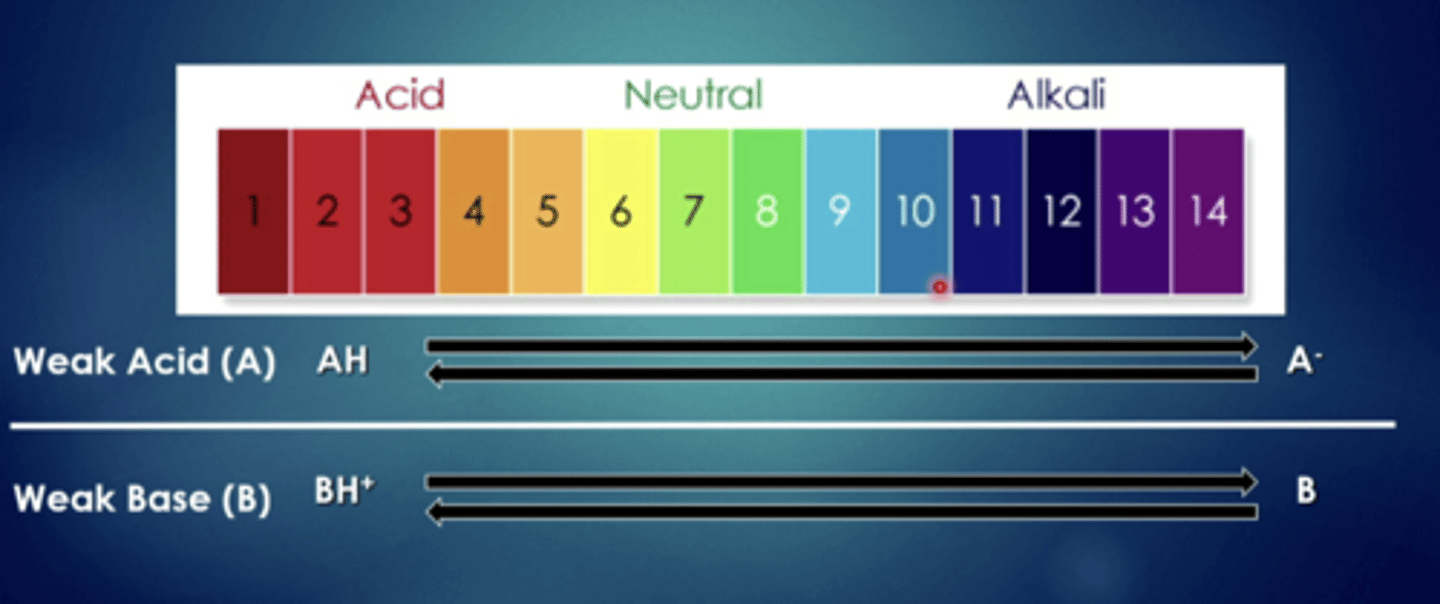
weak acids can cross easily when they are in ....
more acidic solutions
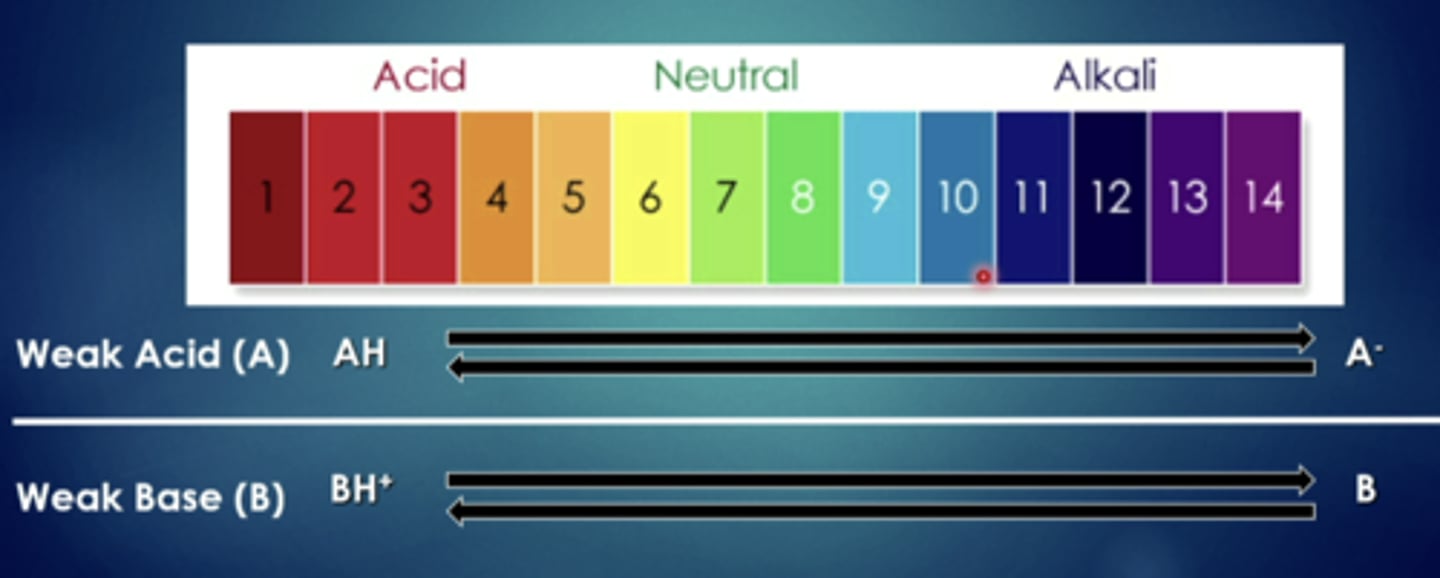
weak acids have a harder time when they are in...
more basic (alkaline) solutions
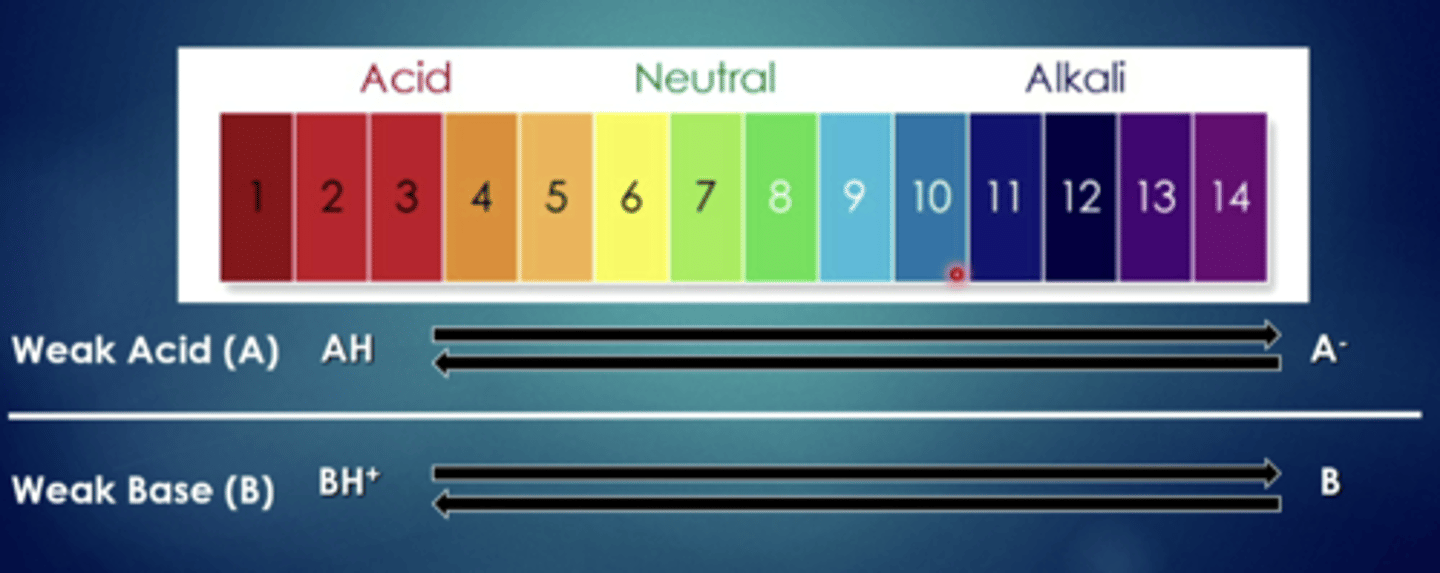
weak bases can cross easily when they are in...
more basic (alkaline) solutions
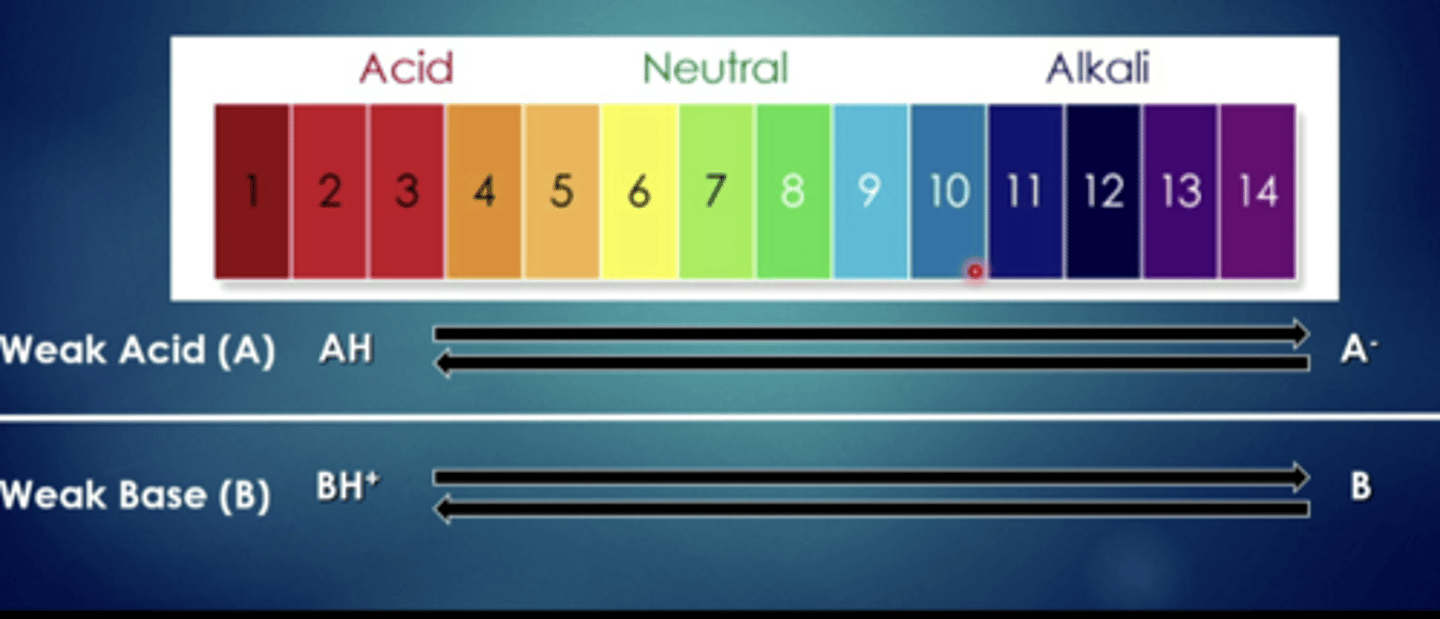
weak bases have a harder time when they are in...
more acidic solutions
ion trapping: high ph differences across tissues - trapping of weak acids/weak bases in specific tissues
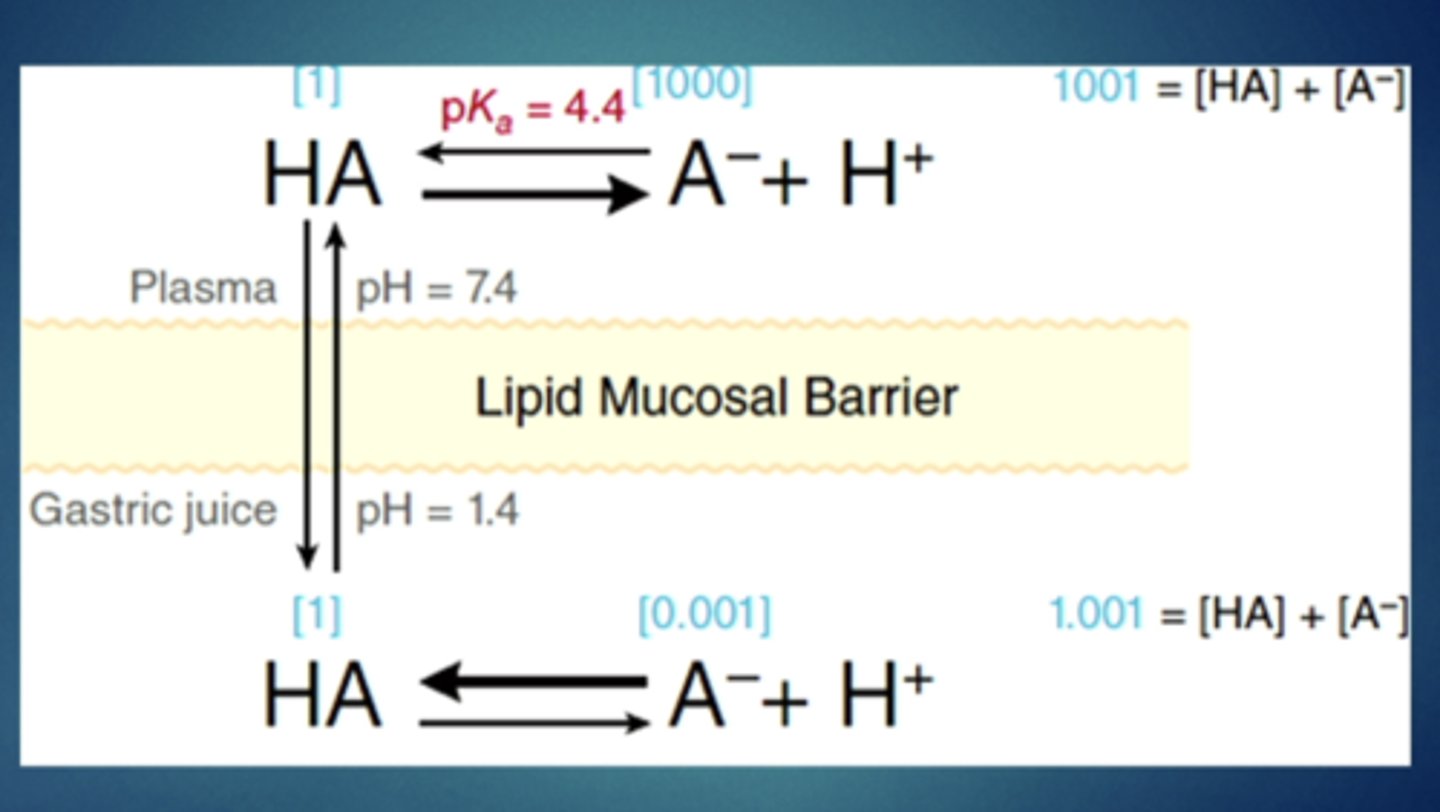
in an acidic stomach, weak ________ will have an easy time crossing the barrier and then they will be trapped and have trouble moving back in
acids
filtration
movement across gaps (capillaries, arteries, veins, glomerulus) for larger molecules that cant cross cell membranes
receptor mediated transport
drug combines with a transport protein in the membrane and the complex allows drug to cross the membrane
two ways: facilitated diffusion and active transport
facilitated diffusion
Movement of specific molecules across cell membranes through protein channels
active transport
Energy-requiring process that moves material across a cell membrane against a concentration difference
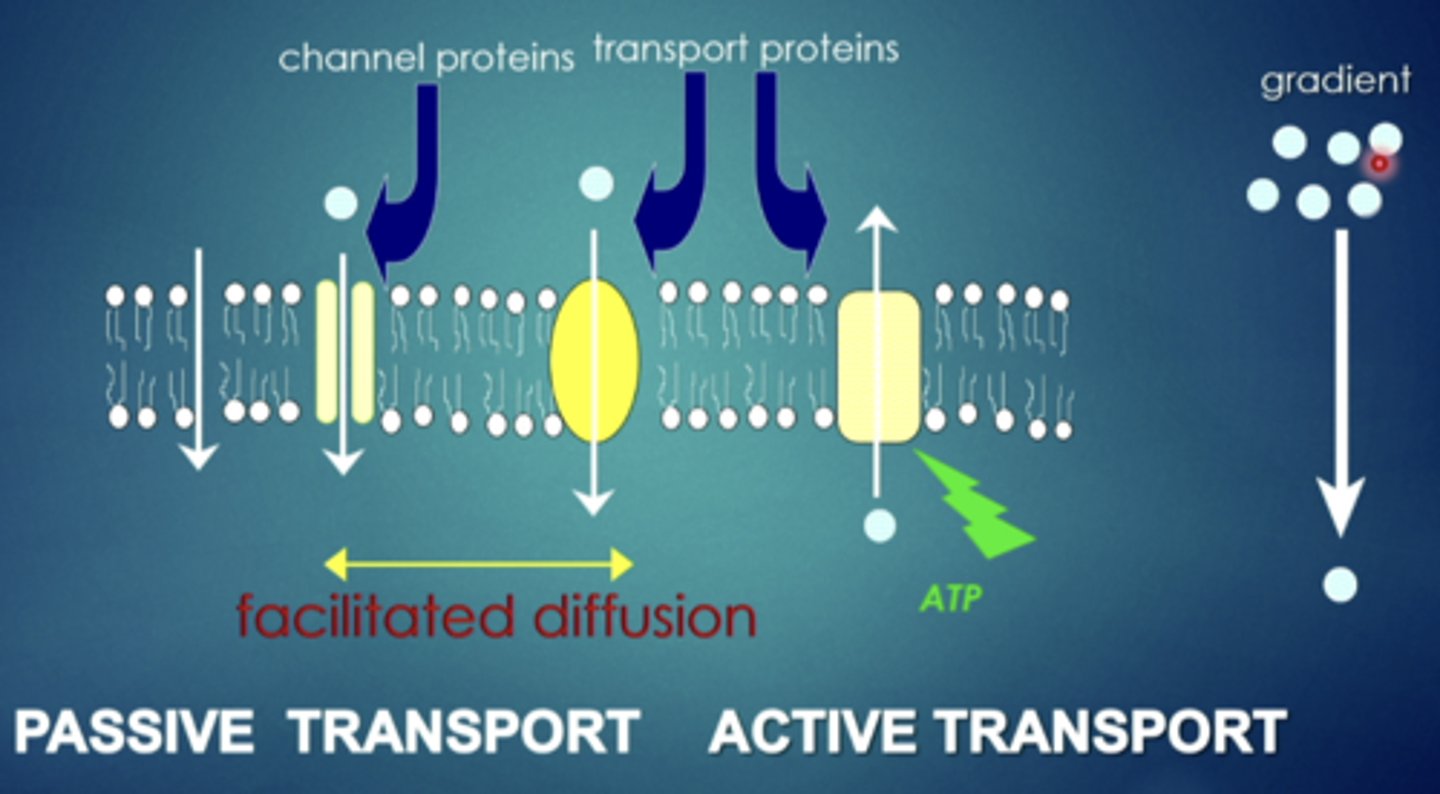
explain this image
some have open channels that allow movement in and out of the cell. some that are specific transport proteins that bind and can move in and out of the cell. some are pumps that require energy and will actively take the drug and move it in and out of the cell in a specific direction
receptor mediated transport - FACILITATED DIFFUSION
primary driving force is chemical gradient of molecule/drug, must move with concentration gradient (no energy), can move molecule across membrane in either direction
receptor-mediated transport - ACTIVE TRANSPORT
requires use of energy by cell (ATP), unidirectional - pumps drug in a single direction, can move AGAINST concentration gradient, usually involves drugs that are highly polar or charged (not very lipid soluble)
absorption
transfer of drugs from site of administration to systemic circulation, intramuscular, intravenous, subcutaneous, oral, etc.
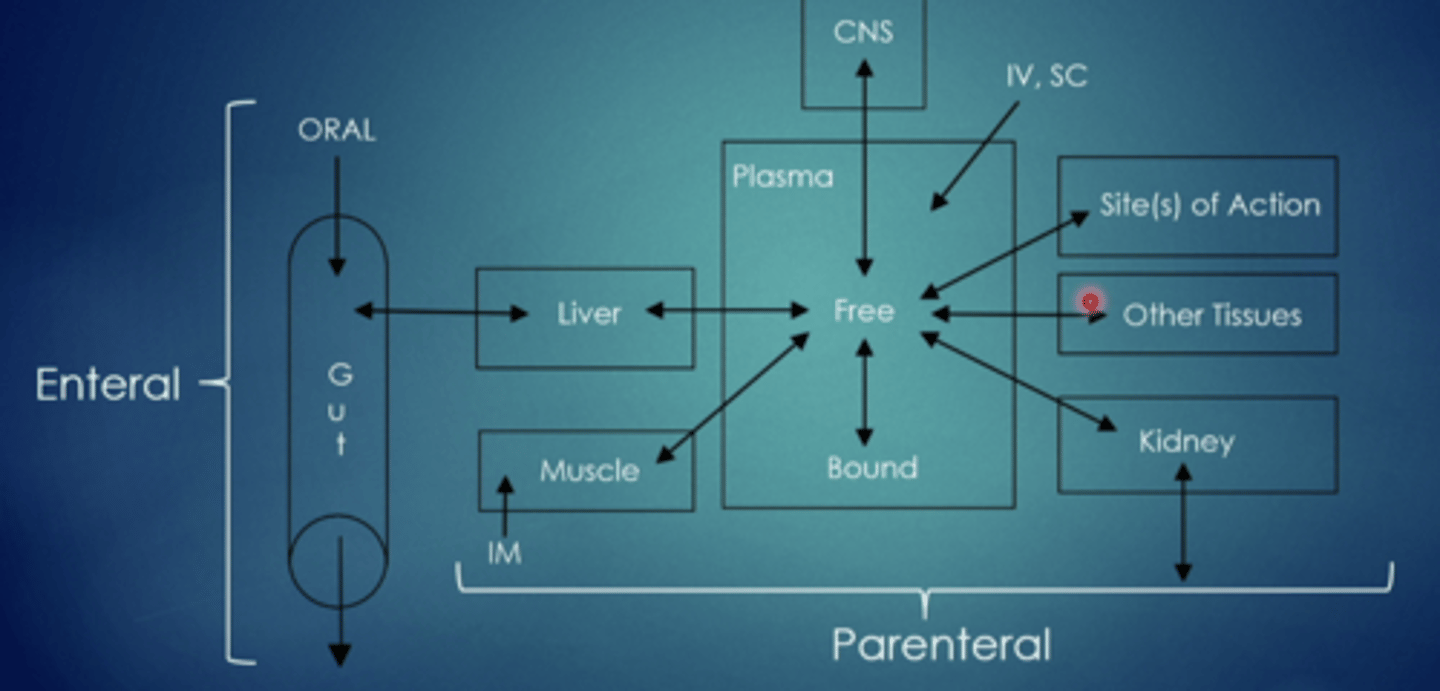
what are the two parts to absorption
enteral, parenteral
enteral
through the GI tract, taken by mouth
parenteral
outside the GI tract
-inhalational - respiratory tract
-subcutaneous
-intramuscular
-transdermal
-intravenous
what are some advantages to enteral administration
safe, convenient, economical
what are some disadvantages to enteral administration
gastric irritation, destruction of drugs in GI tract, irregularities in absorption, requires patient cooperation, FIRST-PASS EFFECT
first-pass effect
drug absorbed from the small intestine is absorbed into hepato-portal circulation and arrives at the liver first (the primary center of metabolism in the body), this means it is possible for a drug to potentially be metabolized in the liver before entering systemic circulation
mouth
small surface area; pH - 6
stomach
pH - 1-2; ion trapping
small intestine
large surface area; pH - 5-8
large intestine
50% of blood supply bypasses liver
more acidic drugs (weak acids) will be absorbed in the ...
stomach
more alkaline drugs (weak bases) will be absorbed in the ...
small intestine
what are some advantages of intravenous administration
no absorption required, can attain desired drug concentration more rapidly, can adjust dosage more readily, can administer irritating solution, bypasses first-pass effect
what are some disadvantages of intravenous administration
no absorption required, risk of infection, pain/difficulties in self-medication, cannot administer drugs that precipitate in blood, and cannot mix drugs in oily vehicles
intramuscular
bigger muscles (buttocks, thigh, calf) absorption by filtration, bypasses first-pass effect, absorption rate can be altered, different absorption rates from different muscle groups
different drugs can ...
slow or increase the rate of absorption
subcutaneous
absorption by filtration, bypasses first-pass effect, slow constant absorption, rate can be altered
-aqueous solution
-suspension in oil
-implanted solid drug
inhalational administration
can be site of administration of both local and systemic effects, diffusion into blood through alveoli, absorption characteristics of lung
-large surface of blood flow
-high amount of blood flow
-leads to almost instantaneous absorption
bypasses first pass effect
what are some disadvantages to inhalational administration
cumberstome administration, difficulty in dosage regulation
transdermal administration
can be used for local and systemic administration, absorption by passive diffusion, limited by degree of hydration
what are some factors that modify absorption
drug concentration at absorption site (high concentration = faster absorption, low concentration = slower absorption)
blood flow at absorption site (high blood flow = rapid absorption, low blood flow = slow absorption)
area of absorbing surface
route of administration
food and gastric emptying
intestinal motility
metabolism/destruction in GI tract
distribution
transfer of drug from systemic circulation to tissues
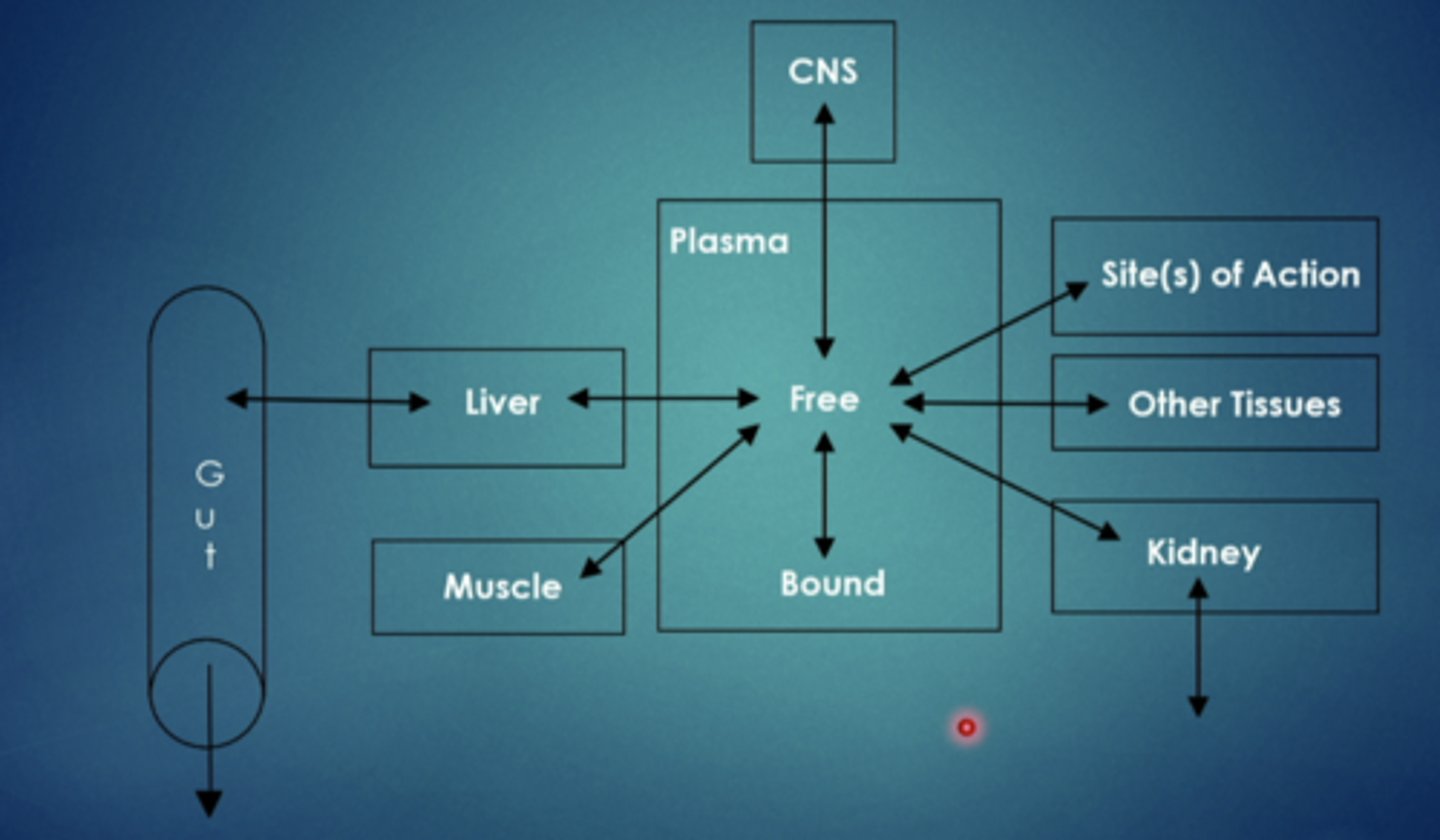
what are some factors that influence distribution
regional blood flow (different parts of the body have different blood flow going to them, heart/brain = high blood flow.....fatty tissue = low blood flow)
capillary permeability
rate of transfer to tissues
binding to plasma proteins
accumulation into tissues (trapping weak acids and weak bases)
plasma protein binding
drug bound to a plasma protein cannot distribute to tissues or be eliminated; the bound drug is pharmacologically inactive. only the free, unbound, form of the drug is available for distribution to sites of action and for elimination. one drug can displace another from binding site
what are different types of plasma proteins
albumin: bind acidic drugs
lipoproteins: bind lipid-soluble drugs
a1-acid glycoprotein: bind basic drugs; induced by trauma, injury, or stress
example test question:
patient is on a drug that binds to plasma albumin, they are about to be administered another drug, it also binds to plasma albumin, what should you do?
reduce the dose of drug x
what are the 3 barriers to drug distribution
blood brain barrier, placental transfer, blood testicular (sertoli cell) barrier
blood brain barrier
formed by capillary endothelial and glial cells, can be altered by inflammation
placental transfer
doesnt prevent transport (600-1000Da), lipid soluble drugs pass freely, problems for fetus
blood testicular (sertoli cell) barrier
prevents drugs from contacting spermatocytes and mature spermatids, inhibit penetration of chemotherapeutic drugs
metabolism
biotransformation of drug into different (more water soluble) form, primarily occurs in the liver but enzymes are all over the body performing metabolic functions
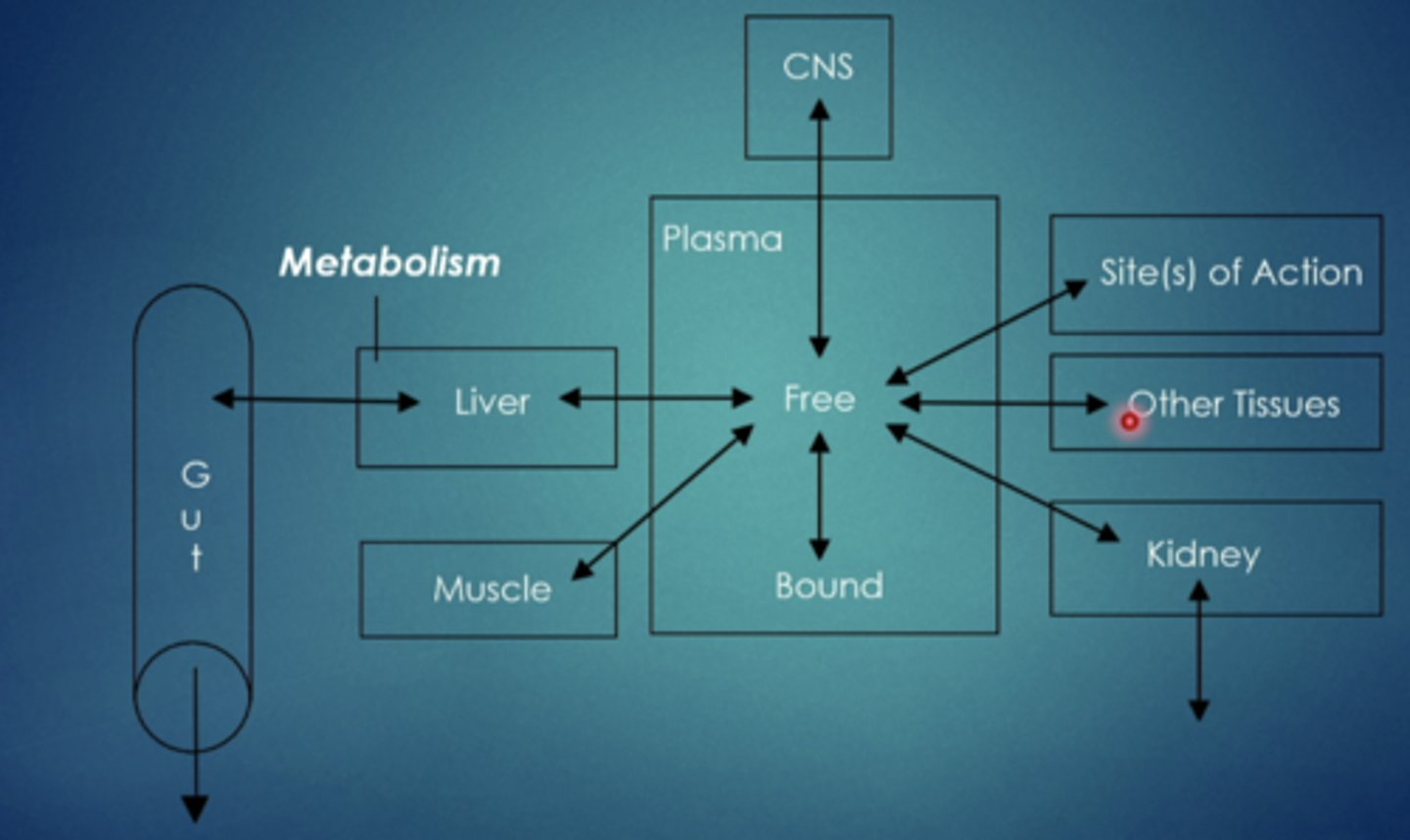
metabolism (biotransformation)
conversion of drug to a different chemical structure, mainly occurs in the liver via a family (system) of liver enzymes - the cytochrome p450 (cyp) system. these enzymes are responsible for phase 1 and 2 reactions
phase 1 reaction
oxidation/reduction reactions by oxygenases and reductases, can occur first but dont have to, simpler transformations - oxidation reduction reactions
phase 2 reaction
formation of conjugates by transferases; drugs are conjugated with a sugar, an amino acid or sulfate,
conjugation reactions
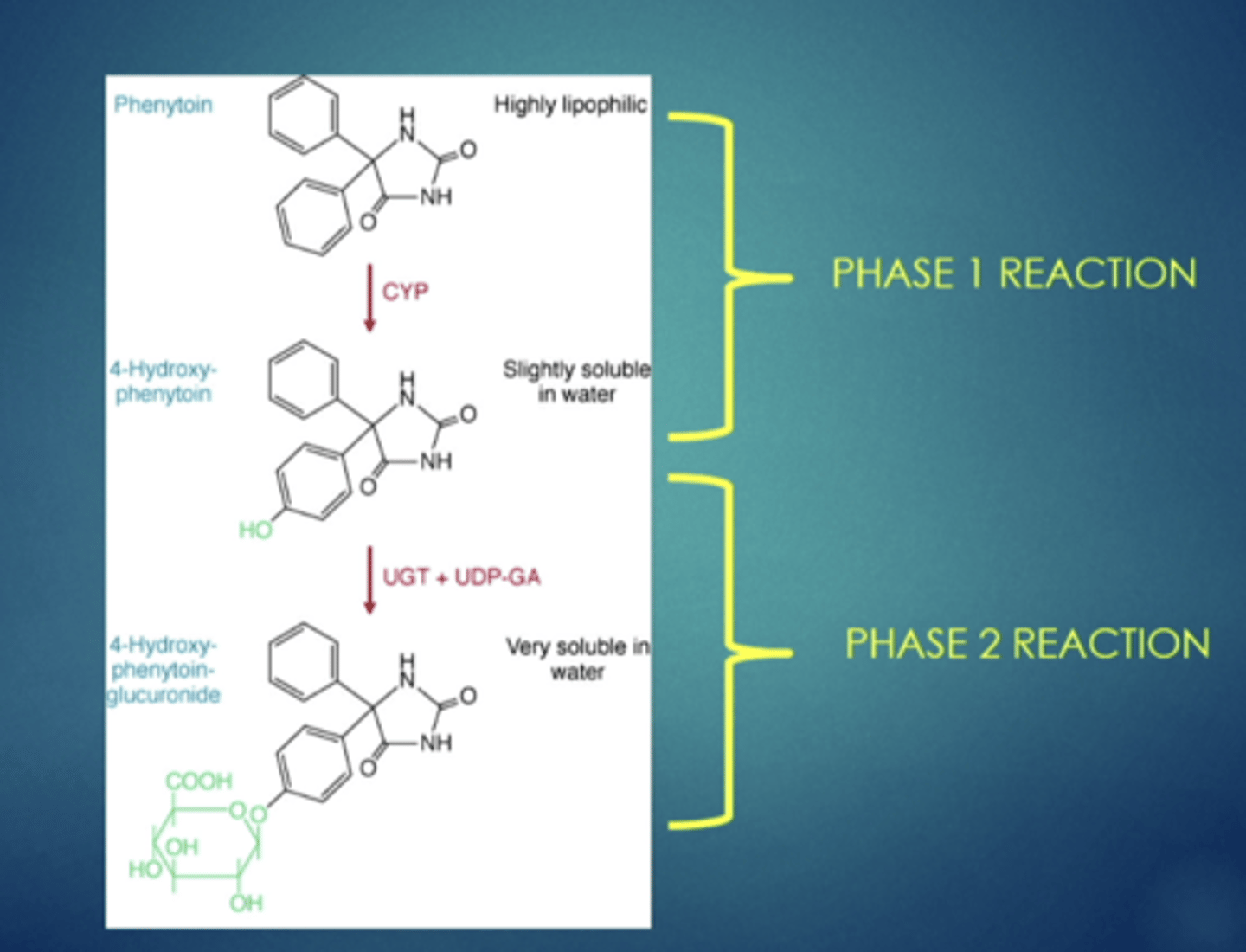
example of metabolism, explain
a drug starts as the first compound, once it goes through a phase 1 reaction a hydroxyl group is added, after it undergoes a phase 2 reaction, from the hydroxyl group, a large glucuronide is added, resulting in the drug becoming soluble, larger, easier to trap, and easily removed in the urine (renal excretion)
what are some consequences of metabolism? metabolites can become:
more water soluble - increased renal excretion
more active (increased therapeutic effect)
--completely active from an inactive drug (called prodrugs)
less active
completely inactive
more or less toxic
---example of drug with toxic metabolite that is clinically relevant: acetaminophen
some drugs are administered pharmacologically inactive and need to be activated via metabolism. these are referred to as ...
prodrugs
why do prodrugs have complications
due to reduced effectiveness if metabolic enzyme is inhibited (drug drug interactions)
examples: clopidogrel (anti-platelet); carbamazepine (anti-epileptic); tamoxifen (hormone chemotherapeutic)
what are some factors affecting drug metabolism
genetics and aging
why can aging affect drug metabolism
very young patients have lower drug metabolism
teens-60 years have a higher drug metabolism
elderly have lower drug clearance
---decreased absorption
---altered distribution
---decreased metabolism
---decreased excretion
excretion
removal of drug from the body, primary route: gi tract and kidney
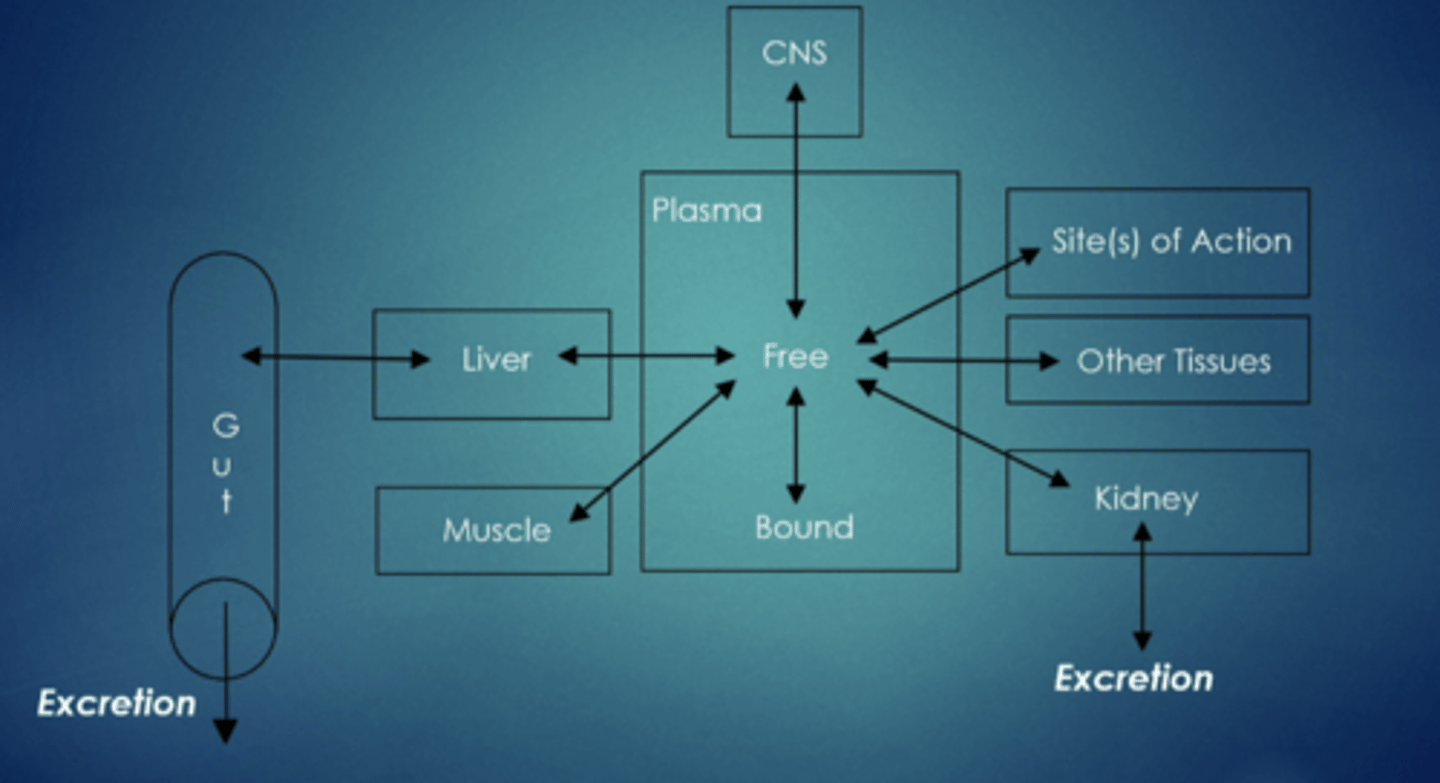
what are other potential routes for elimination
GI tract (secretion via liver into bile)
kidney (urine)
sweat glands
respiratory tract (exhaled air)
saliva
tear duct
hair
mammary glands (milk production)
hepatic/gastrointestinal elimination
secretion into bile and small intestine
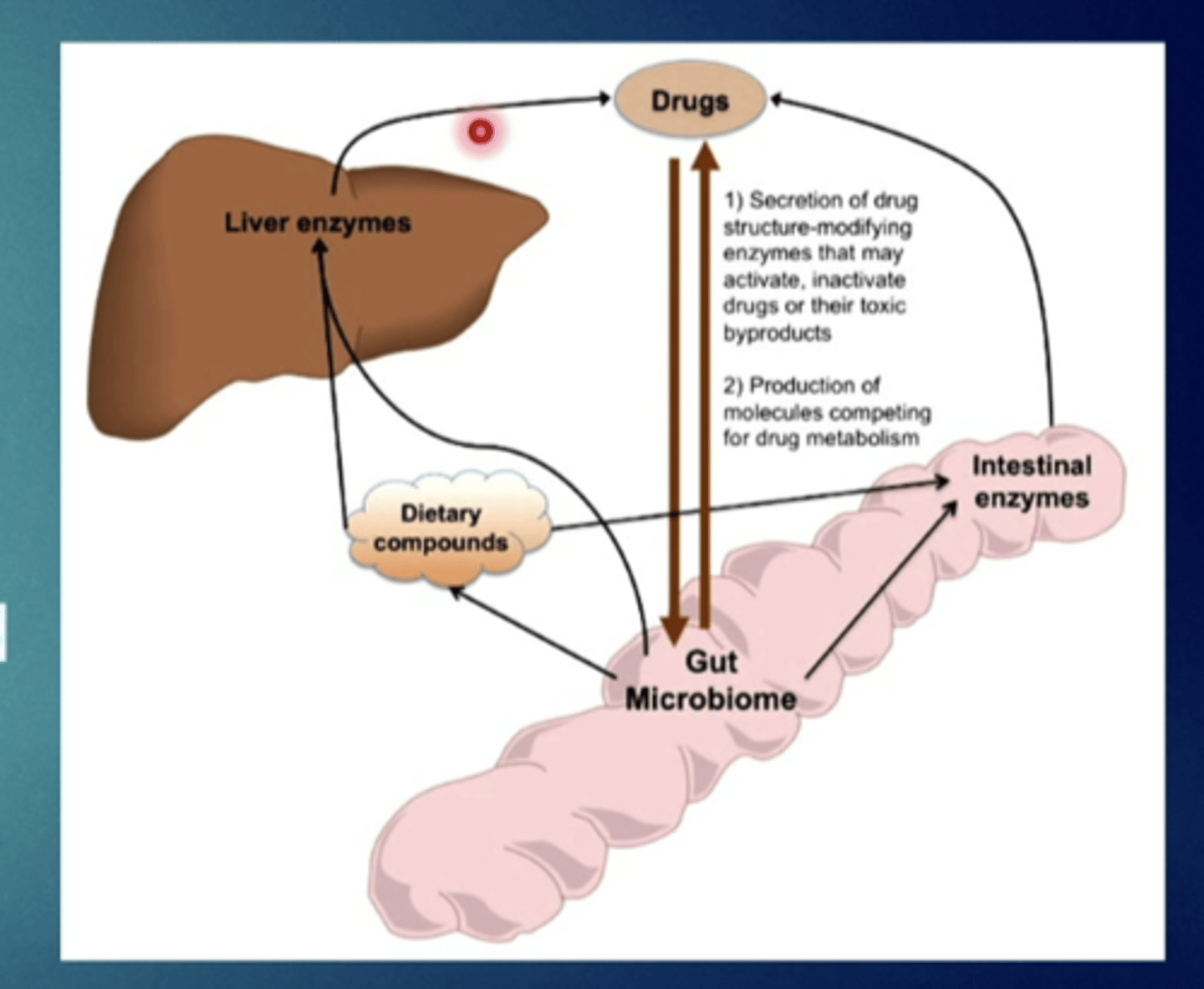
enterohepatic cycling
drug conjugate secreted into the bile and reconverted to parent compound by intestinal bacteria can be reabsorbed from the small intestine - recycling
consequence of hepatic/GI elimination:
alteration of the intestinal flora can affect the ....
action of some drugs
what is the consequence of enterohepatic cycling
it increases drug duration of action, drug half life
renal elimination
glomerular filtration - hydrostatic pressure
active secretion to proximal tubule
passive reabsorption from distal tubule back into systemic circulation
drugs can passively diffuse from urine into tubular cells and back into blood stream - recycling
---ph of urine and pKa of drug are important
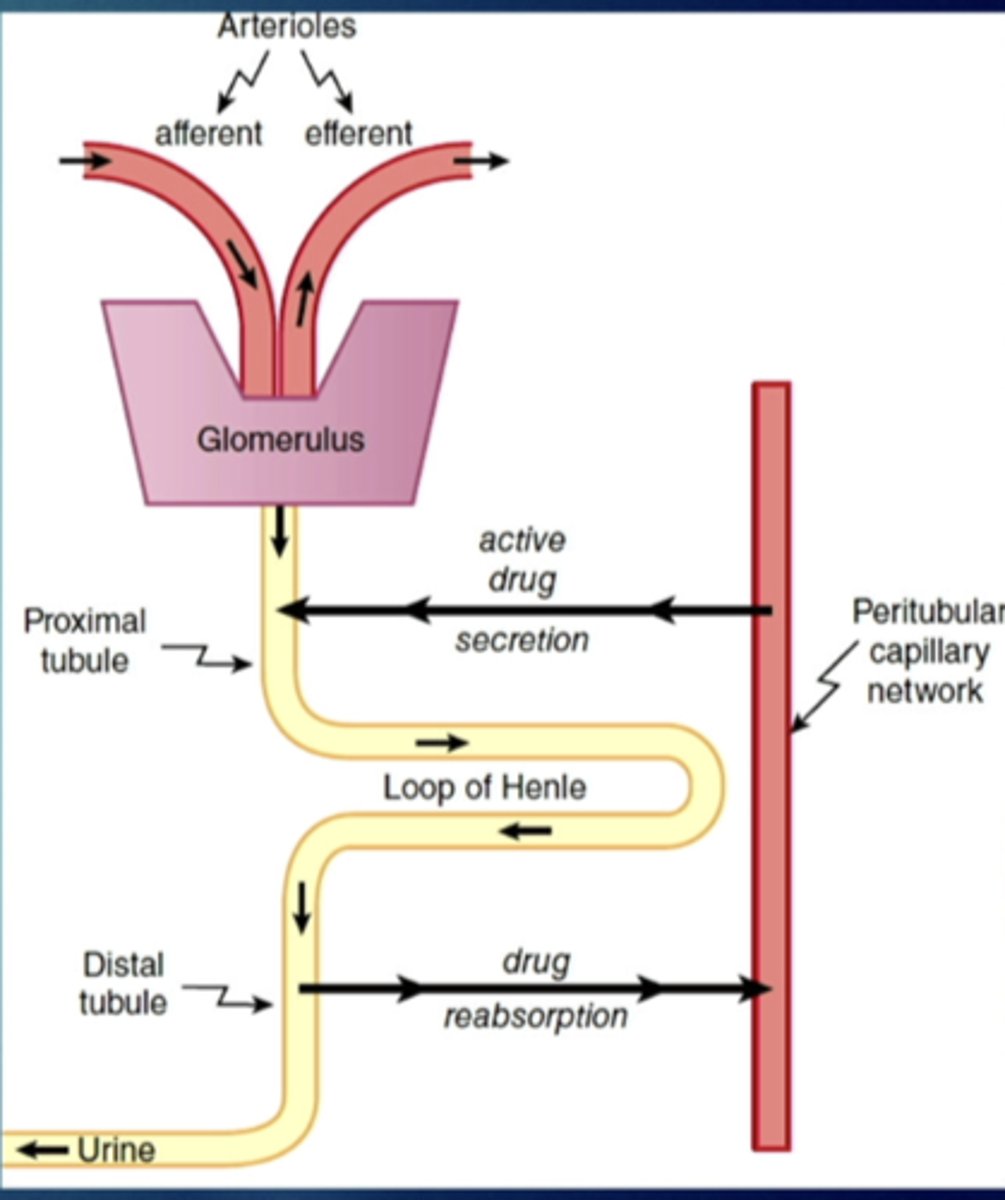
what influence does urinary ph have on renal elimination
can affect excretion of weak acids and weak bases
more alkaline urine will result in greater excretion of weak acids/reabsorption of weak bases
-forced alkalinize diuresis
more acidic urine will result in greater excretion of weak bases/reabsorption of weak acids
-forced acidification diuresis
forced alkalinize diuresis
administer bicarbonate or use of diuretic such as acetazolamide will increase bicarbonate in urine and make it more alkaline
forced acidification diuresis
administer ammonium chloride
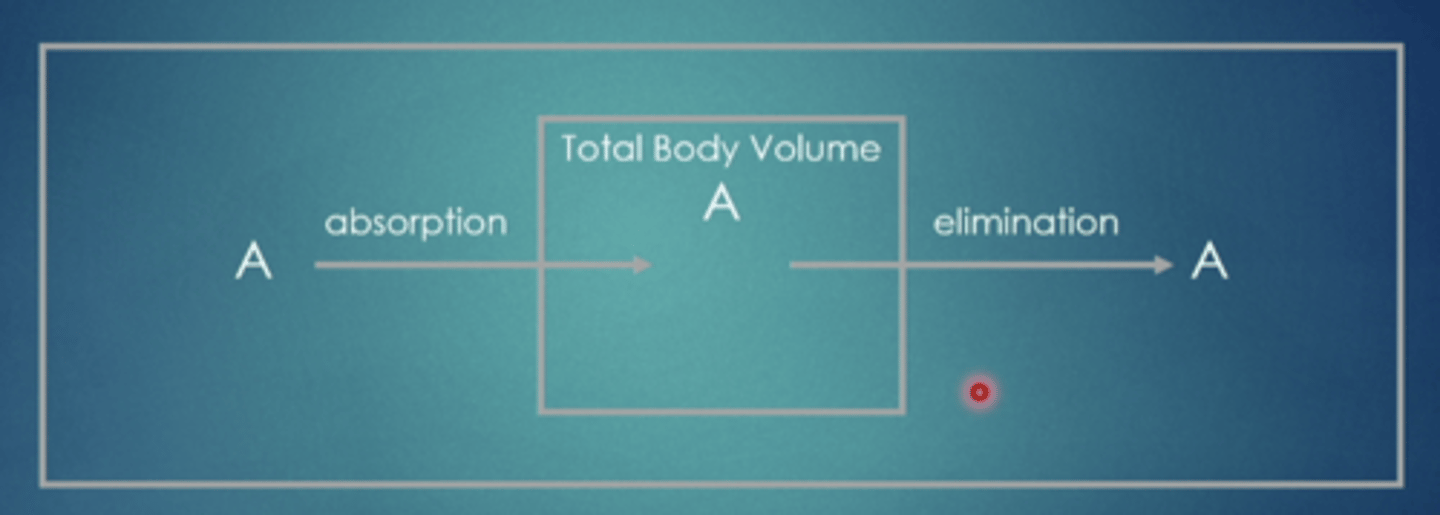
explain this image
drug goes into the body, circulates through, and then is eliminated
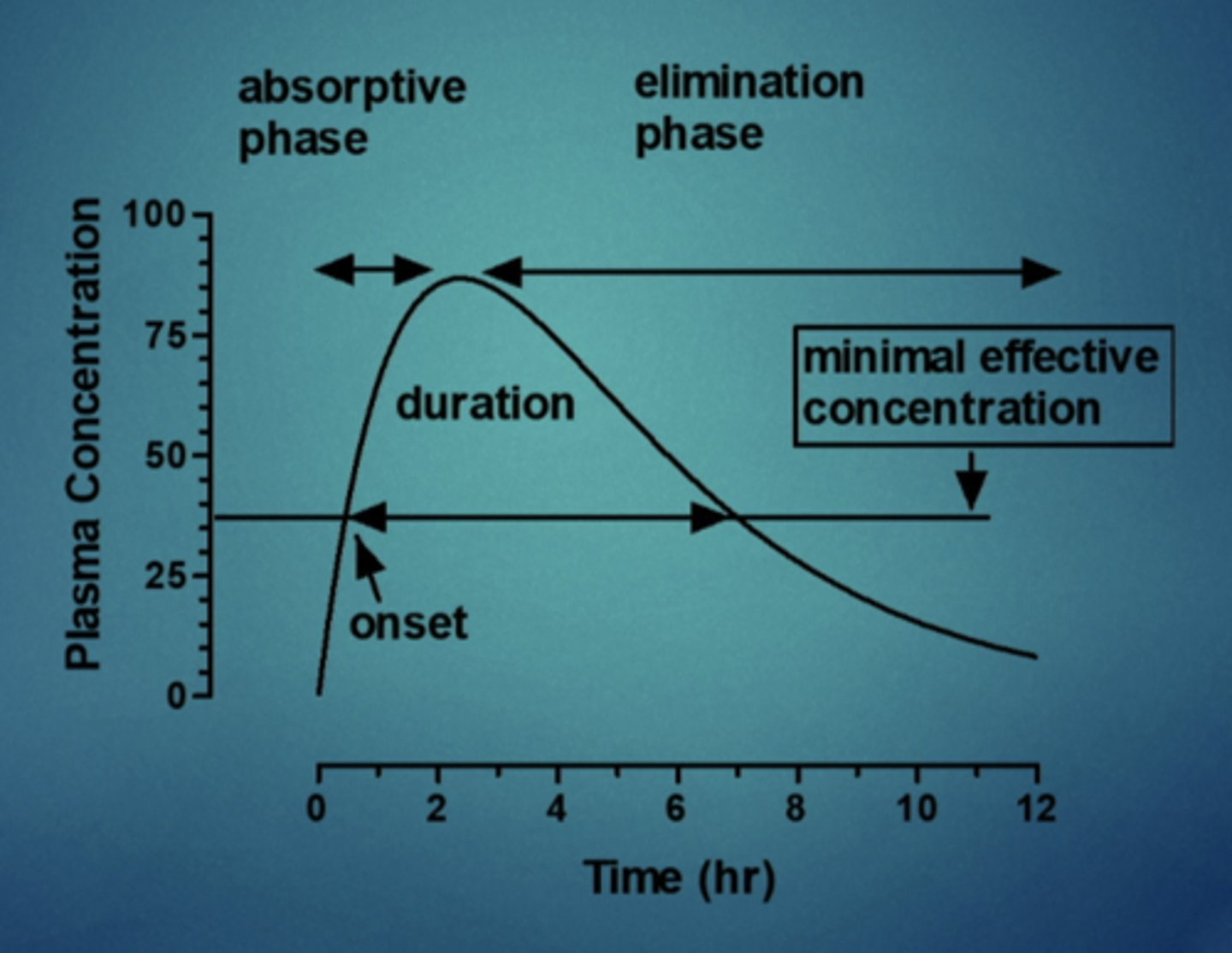
explain this image
you have time for a given drug and time, time is 0 bc drug was just administered, overtime it gradually is absorbed into systemic circulation into plasma, and it gradually increases until it plateaus, more is being eliminated than absorbed, concentration decreases over time until it gets back down to 0. if you ask the patient when they feel the drug you can mark onset of the drug (15 minutes) so plasma concentration is about 35, so anything above this point, they are feeling the drug. drug duration is 7 hours
first order elimination
rate of elimination proportional to concentration of drug remaining in body
constant percentage of drug eliminated per unit time (ex: 50%/4 hr)
plasma half life (t1/2)
time necessary to reduce plasma levels by one half
what is the consequence of first order elimination
drug fully eliminated from body in set amount of time regardless of starting dose (time based on drug half life)
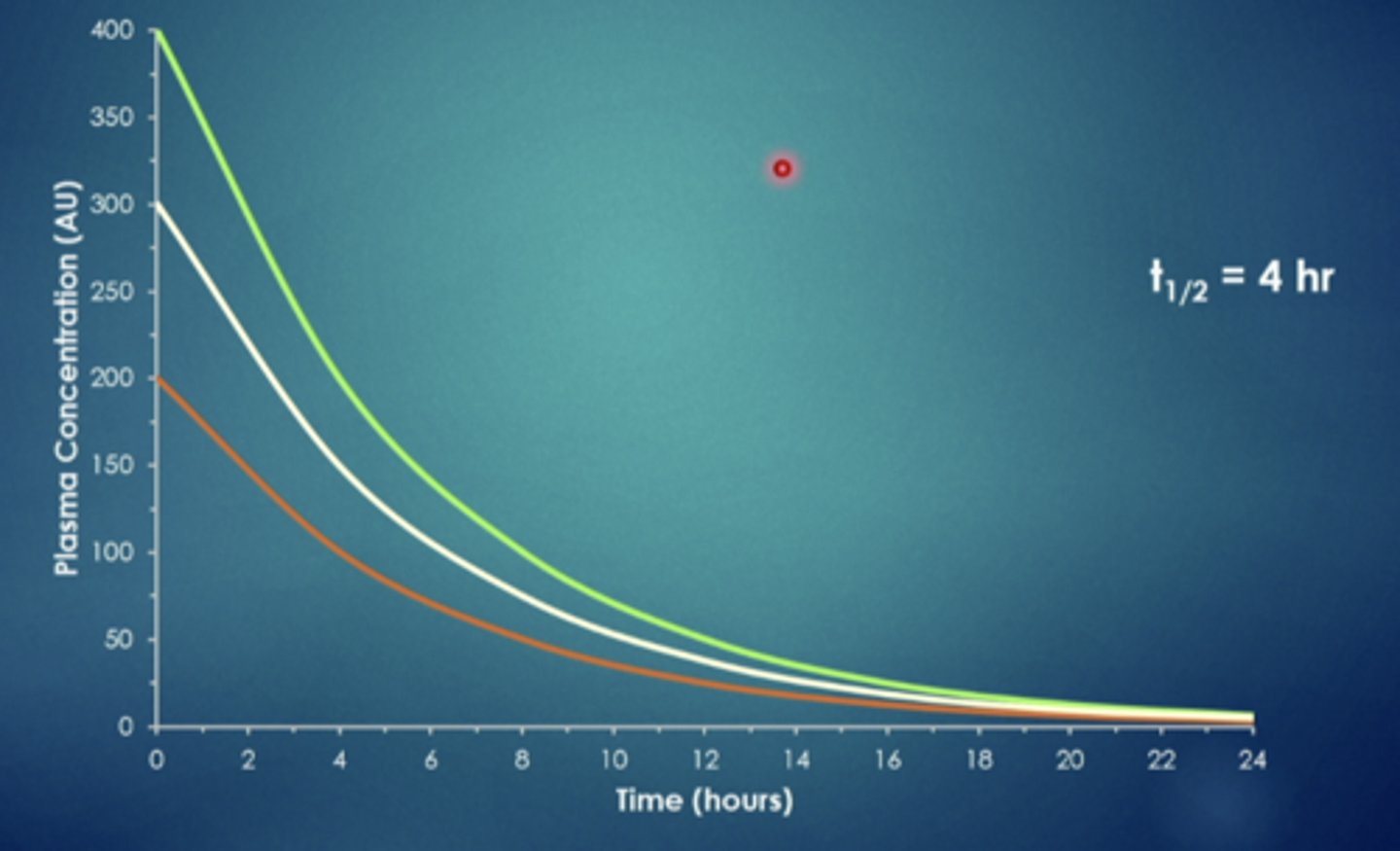
explain this image
if you administer a drug intravenously it will look like this. this drug has a half life of 4 hours, so every 4 hours half of it is eliminated, regardless of the starting dose, after the certain amount of time they will all reach a point and is eliminated at the same time
first order elimination multiple dosing
impact of dosing frequency and dose
both example dosing regimens give the same average plasma concentration over time
however the blue curve has much more variability in hour to hour plasma concentration
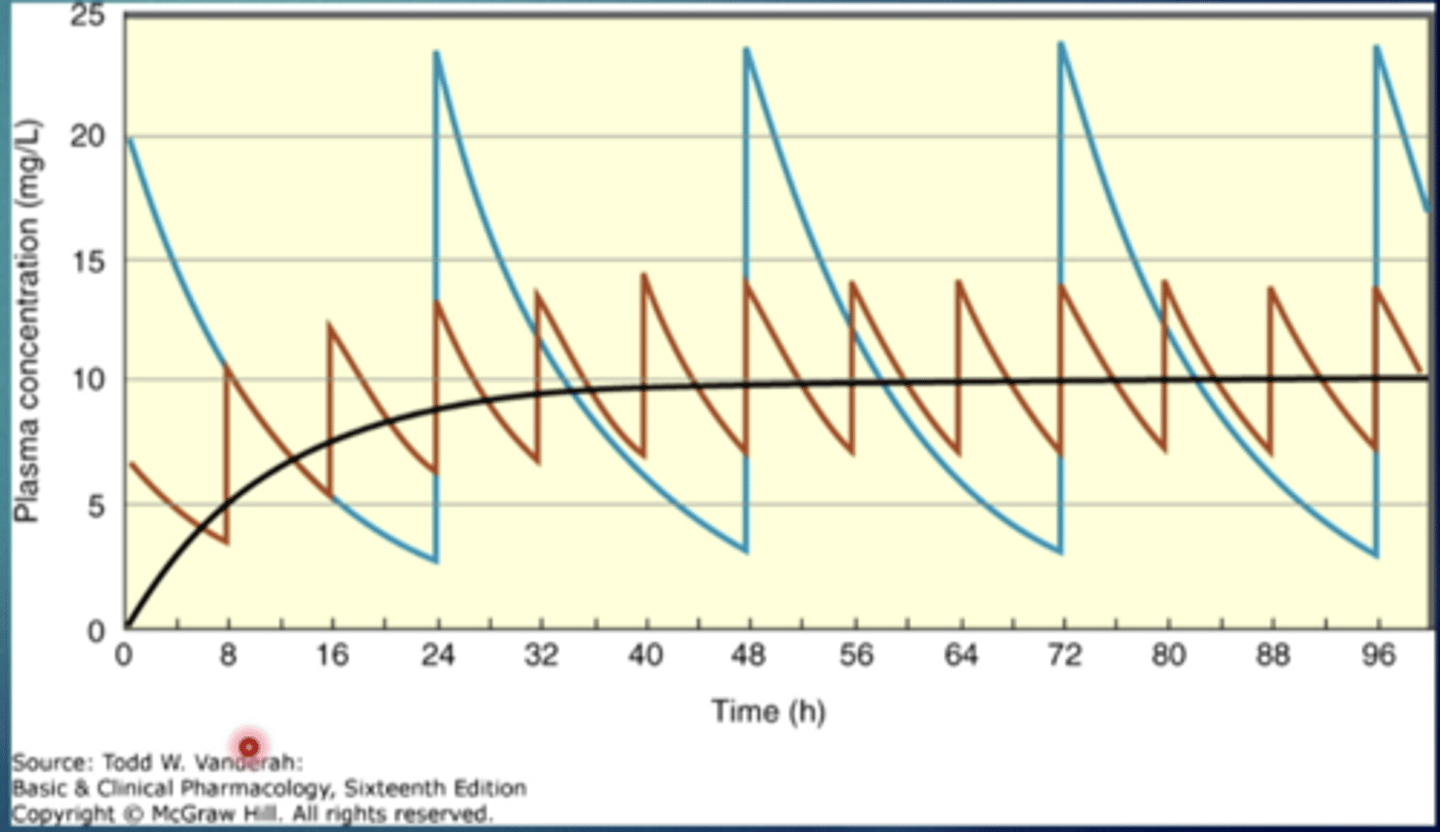
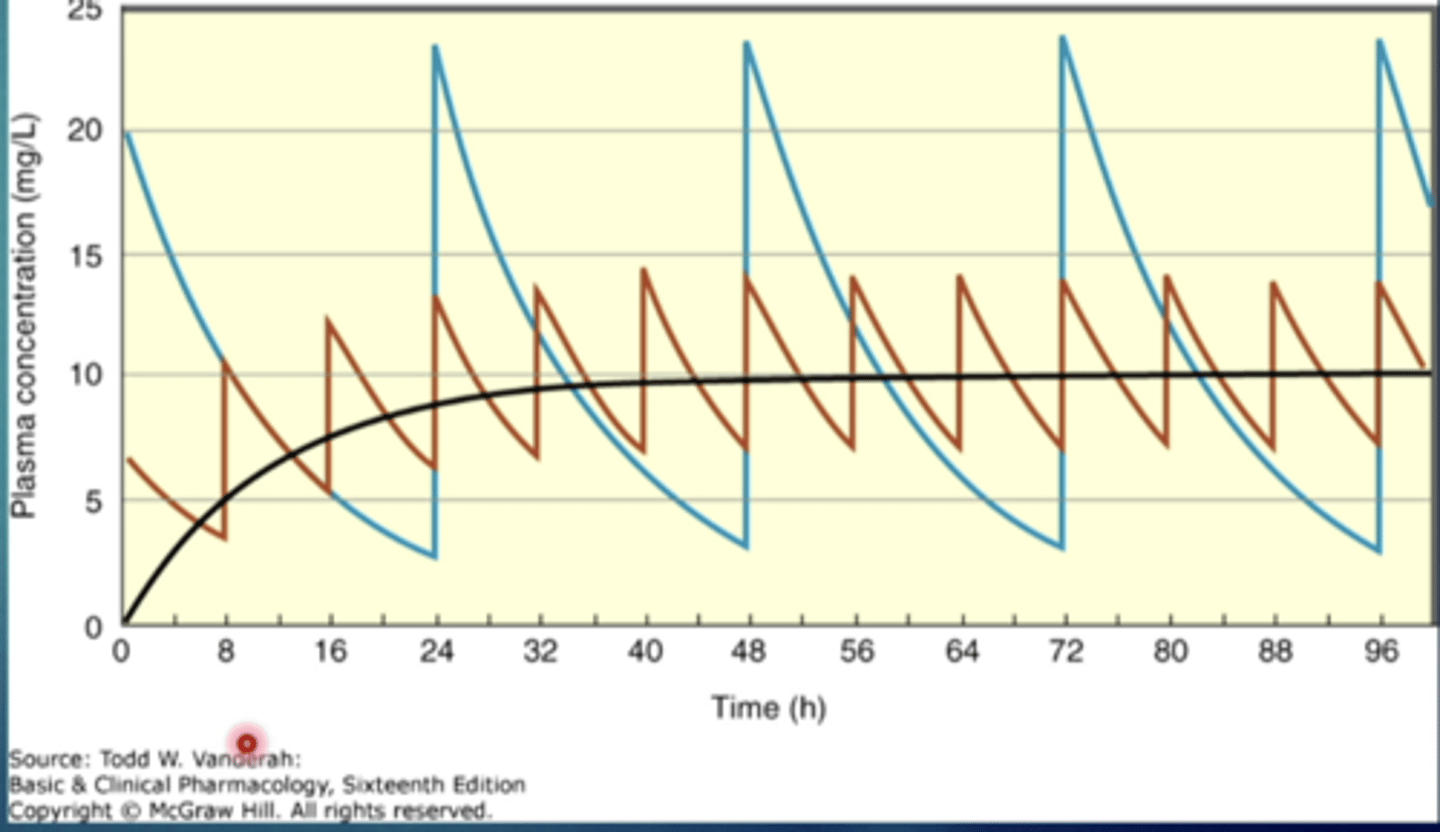
explain this image
both lines represent a drug with the same half life
the blue line: drug being administered less frequently with a larger dose
the red line: drug being administered more frequently with a lower dose
zero order elimination
also referred to as rate limited elimination
constant rate of elimination irrespective of plasma concentration
constant amount of drug eliminated per unit time (ex: 50mg/4hr)
most notable example is ethanol
what is the consequence of zero order elimination
time to fully eliminate depends upon initial dose (no half life)
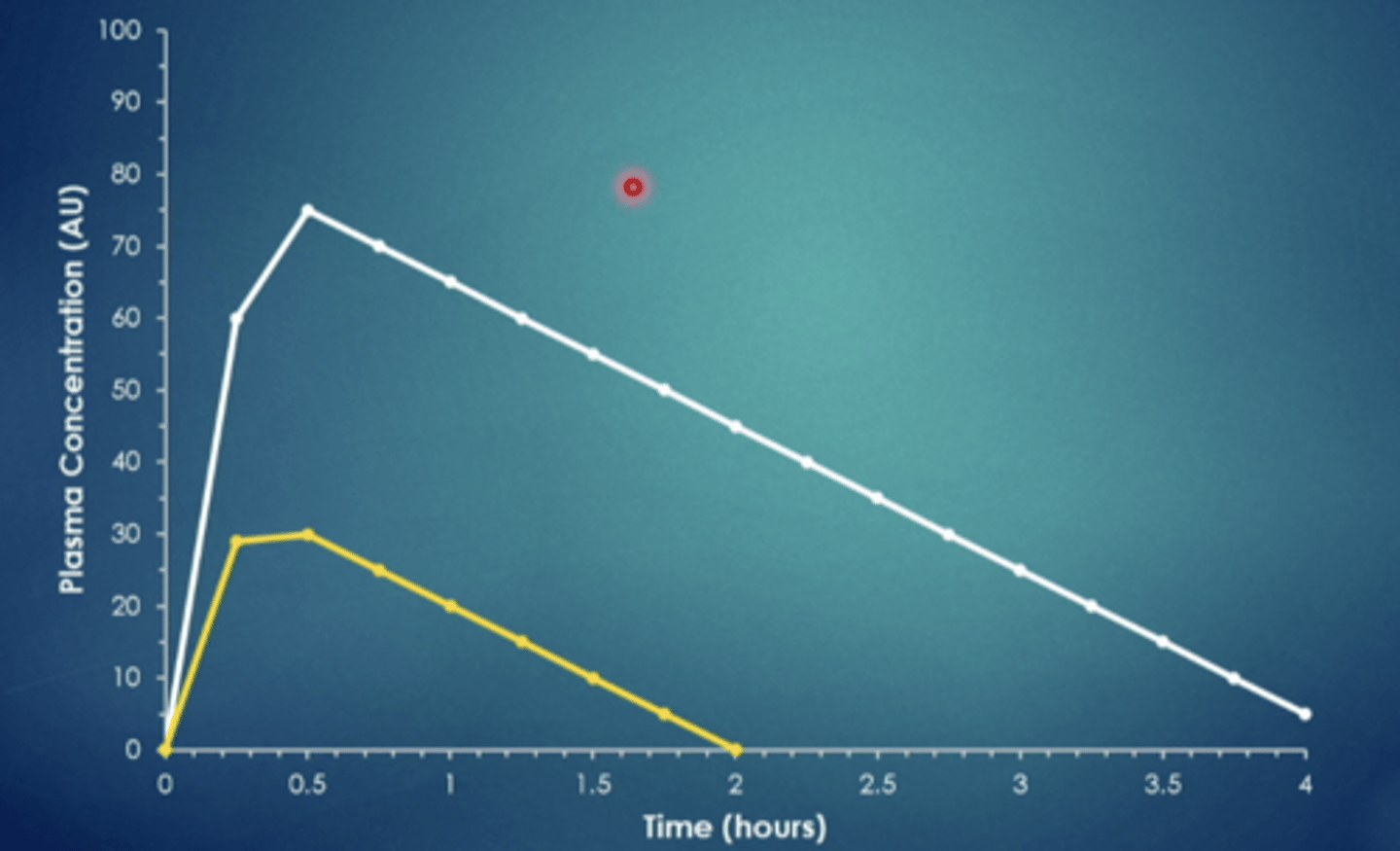
explain this image
the time to eliminate depends on the amount taken. double dose, double time to eliminate. same drug in the image just different dose