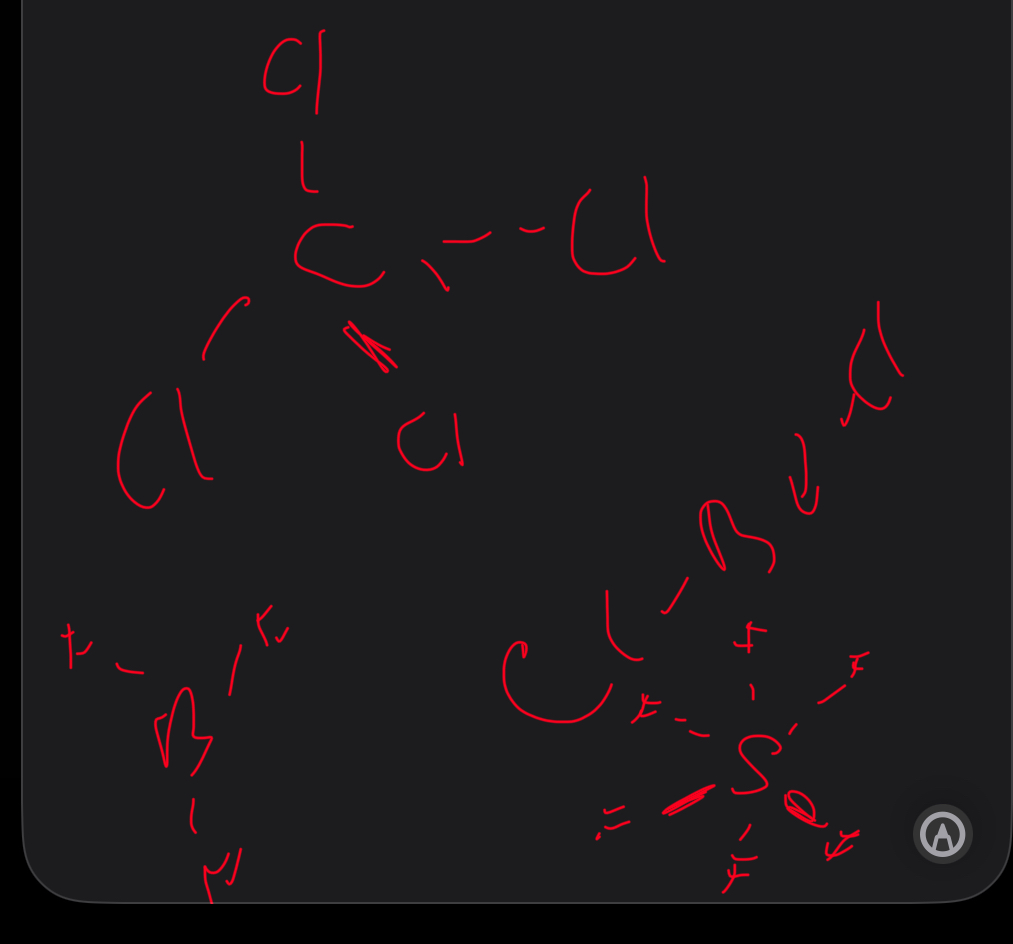
3.1.3.6 bond polarity
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
11 Terms
What is electronegativuty
Power of an atom to attract the pair of electrons in a covalent bond
What is the electron distribution like in a covalent bond betwee different elements and what does it produce
Unsymmetrical
It produces a polar covalent bond and may cause a molecule to have a pemanent dipole
What does electronegativity depend on
Nuclear charge
Sheilding
Distance from nucleus
What is the trend in electrongetaiviy up a group
E.n increases
Atoms get smaller and there is less sheilding
What is the most electronegative element
Fluorine
Why does electronegativity increase acr0s period
Atomic radius decreases so stronger attraction between positive nucleus and 2 electrons in covalent bond
Nuclear charge increases and so greater attraction
If there is no difference o very small difference in E.N then the compound is
Non polar for example cl2
A very large difference is e.n results in
Ionic compound
E.g NaCl
Give an example of a molecule that has polar bonds but is non polar
Carbon dioxide
This is because polarities are equal (arranged symmetrically and both are c=o so Polarities cancel out
Give. 4 examples of different molecules that cancel out