Food physics
1/61
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
62 Terms
What are the 5 essences humans have?
●Colour
●Touch
●Smell
●Taste
●Sound
What connects molecular properties to macroscopic properties in food physics?
emperature, density, type of molecule, external energy provided, and time.
What are molecular properties in food physics?
Properties at the scale of atoms and molecules, such as intermolecular forces, diffusion, water binding, and molecular conformations.
What are macroscopic properties in food physics?
Properties observable at the bulk scale, like texture, viscosity, elasticity, density, and heat transfer.
What is microstructure in food physics?
Microstructure refers to structures on the micron scale (e.g., fat globules, air bubbles, protein networks). It describes how these elements are organized, and this organization directly affects macroscopic properties like texture, appearance, and mechanical strength.
What is mesostructure in food physics?
Mesostructure is the intermediate scale between the molecular and macroscopic world. It describes how different molecules assemble into larger structural forms (spherical, planar, cylindrical), creating a bridge between individual molecular interactions and the bulk properties we perceive in food.
what acts as a stabilizer between oil and water?
protein
What is the dispersed phase in a food system?
The dispersed phase is the material that exists as small, separate particles or droplets within another medium. It is the “tiny bits” that are spread throughout the system.
Examples:
Oil droplets in mayonnaise (emulsion)
Air bubbles in whipped cream or meringue (foam)
Sugar crystals in candy syrup (suspension)
Solid cocoa particles in chocolate (suspension)
What is the dispersing in a food system?
The dispersing phase (continuous phase) is the medium that surrounds and carries the dispersed phase. It’s the “matrix” in which the tiny particles or droplets are distributed.
Examples:
Water in mayonnaise (disperses oil droplets)
Liquid sugar in candy syrup (disperses sugar crystals)
Air in bread dough (disperses gluten and water bubbles)
Water or milk in whipped cream (disperses air bubbles)
What is the range of order in solids, liquids, and gases?
Solid: Long-range order (regular, repeating structure, e.g., crystal lattice)
Liquid: Short-range order (molecules are close but not in a fixed pattern)
Gas: No order (molecules move freely, randomly distributed)
How can you change the order of a material at constant volume?
By changing temperature or pressure:
Increasing temperature → usually decreases order (melting, vaporization)
Increasing pressure → can increase order (compressing gases into liquids or solids)
How can you change the pressure of a material at constant temperature?
Decrease volume at constant concentration → molecules are closer → pressure increases
Increase concentration at constant volume → more molecules in the same space → pressure increases
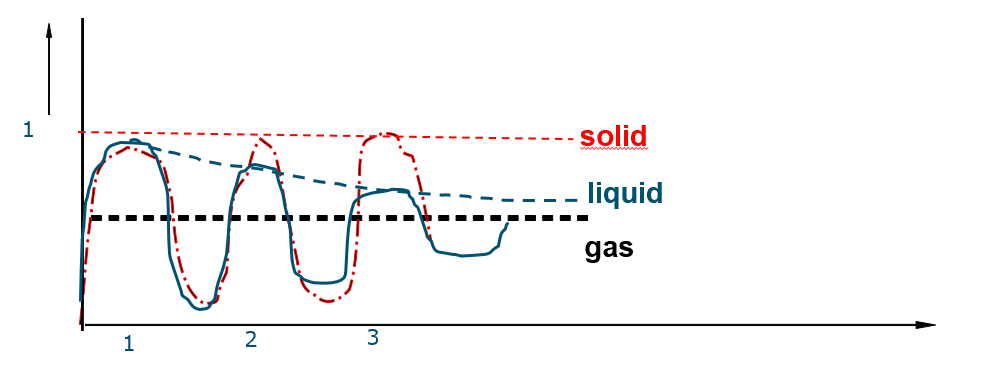
How does the graph for the measure of order look? explain
Solid:
The solid curve exhibits sharp, distinct peaks at regular intervals. This indicates a high degree of order and long-range correlation in a solid, where molecules are arranged in a fixed, repeating lattice structure. The peaks correspond to the shells of neighbouring molecules at specific distances from the origin molecule.
Liquid:
The liquid curve shows a few initial peaks, but these peaks are broader and less defined than those in a solid, and they quickly diminish with increasing distance. This signifies a short-range order in liquids, where molecules have some local correlation due to close packing but lack the long-range order of a solid. The correlation over locations of a molecule declines for a liquid compared to a solid.
Gas:
The gas curve is essentially flat, showing no significant peaks or troughs beyond the very immediate vicinity of the origin molecule. This indicates a complete lack of correlation in a gas, where molecules move randomly and independently with no fixed positions relative to each other.
order of magnitude of water content in cucumber
●99%
order of magnitude of water content in yougurt
90%
Formula and definition of relative humidity
RH is the fraction of the maximum water vapour the air contains:
• RH = 100% air is saturated → condensation can occur.
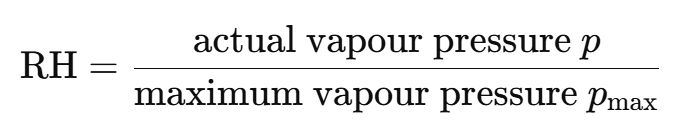
Why does condensation occur on a glass window at night?
• The inside part of the window is colder
than the air in the room.
• Cold surface lowers the temperature of
nearby air, reducing the maximum
vapour pressure (pmax) it can hold.
• When the actual vapour pressure p
exceeds Pmax at the cooler surface,
water condenses → tiny droplets form on
the glass.
What is maximum vapour pressure and how is it determined?
Maximum vapour pressure (Pmax) is the highest pressure water vapour can exert at a given temperature before condensation occurs.
Dependent on temperature: higher T → higher pmax
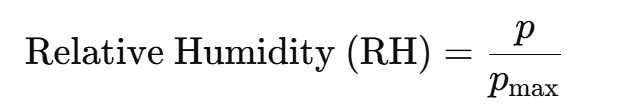
What is water activity (aw) and how is it defined?
Water activity measures the “available” water in a product for chemical reactions or microbial growth.
Pproduct = equilibrium vapour pressure due to the product
Pmax = maximum water vapour pressure at that temperature
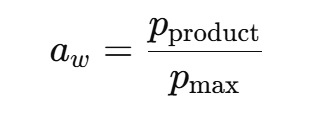
How can water activity (aw) be measured?
Place product in a closed container
Measure RH of air around the product after equilibrium
At equilibrium:
RH of surrounding air (fraction)

What are the two different types of water? and when is it not justified? not that important
free and bound
§free is supposedly more reactive and allows microbes to grow, while bound water is not reactive at all.
Water molecules can not be distinguishable from one another in equilibrium, since if they would, there would be an energy difference between them, implying non-equilibrium
Which structure is relevant in the product case of a zero fat spread?
Planar Structure
What kind of structures can certain lipids like mono-glycerides form, and how do they affect the product?
Form planar structures of micron size.
These layers organize the lipid and water phases, creating a stable microstructure that controls:
Texture (e.g., creaminess or firmness)
Mechanical strength of the product
Distribution of water and other components
How do charged lipids affect planar structures?
They increase the distance between planar layers, allowing lightweight but stable structuring.
Why do some lipid layers become stiff?
Stiffness arises from long chain lengths in the lipid molecules, providing mechanical rigidity.
What determines the water layer thickness between lipid planes?
The charge of the lipid, e.g., DATEM, affects the spacing of water layers in the structure.
What happens when RH of air < aw of a product?
Water moves from the product to the air → drying occurs.
Condition for drying: RH < aw
Water transport continues until RH = aw (equilibrium)
What happens when RH of air > aw of a product?
Water moves from the air to the product → soaking/rehydration occurs.
Condition for soaking: RH > aw
Water transport continues until RH = aw (equilibrium)
How do charged lipids affect planar structures, and what is the impact on the product?
Charged lipids increase the spacing between planar layers.
Effect on product: Creates a lighter, more aerated structure without losing strength, improving weight efficiency and stability.
How does stiffness in lipid layers arise?
from long chain lengths in the lipid molecules, providing mechanical rigidity.
What determines the water layer thickness between lipid planes?
The charge of the lipid, e.g., DATEM, affects the spacing of water layers in the structure.
Controls hydration and spacing between layers, affecting texture, stability, and ability to incorporate other ingredients.
What kind of structures can certain lipids like mono-glycerides form, and how do they affect the product?
Form planar structures of micron size.
These layers organize the lipid and water phases, creating a stable microstructure that controls:
Texture (e.g., creaminess or firmness)
Mechanical strength of the product
Distribution of water and other components
What are the main structures in milk and their typical sizes?
Fat particles: 5–10 μm
Casein micelles: 0.1 μm
Whey proteins: ~1 nm
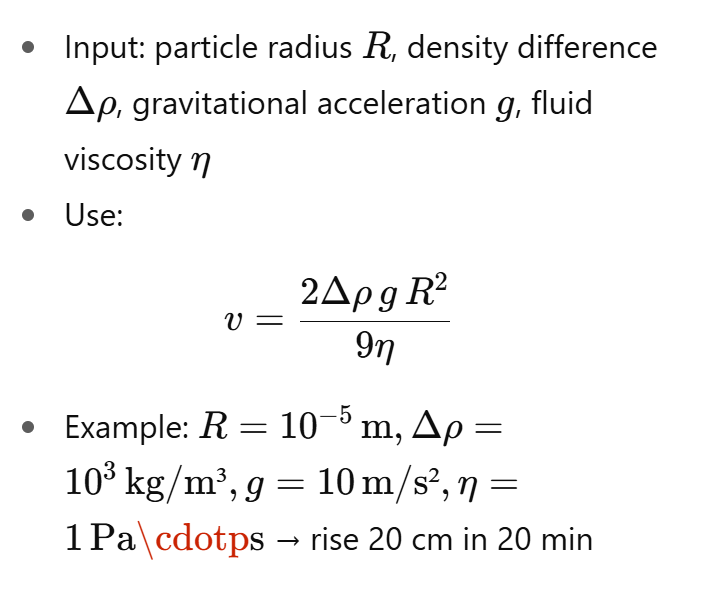
Why do fat droplets in milk cream (rise) and what affects the speed?
Fat droplets are less dense than water → buoyancy causes upward movement.
Velocity increases if the fluid is less viscous.
Upward force due to gravity
R = droplet radius, Δρ = density difference, g= gravity
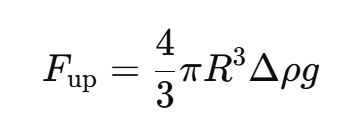
How does milk composition influence creaming?
Higher fat content → more droplets can cream
Thicker milk (higher viscosity) → slows creaming
Smaller droplets → slower creaming (because R3 in force formula)
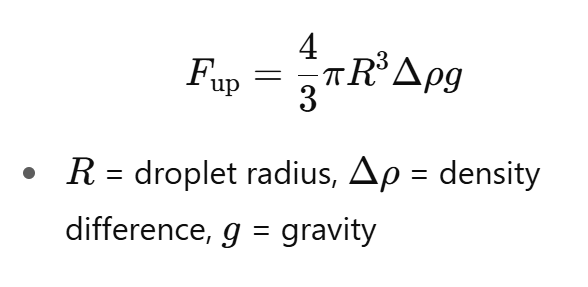
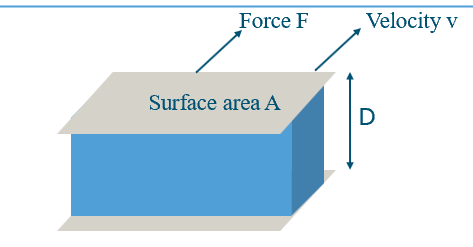
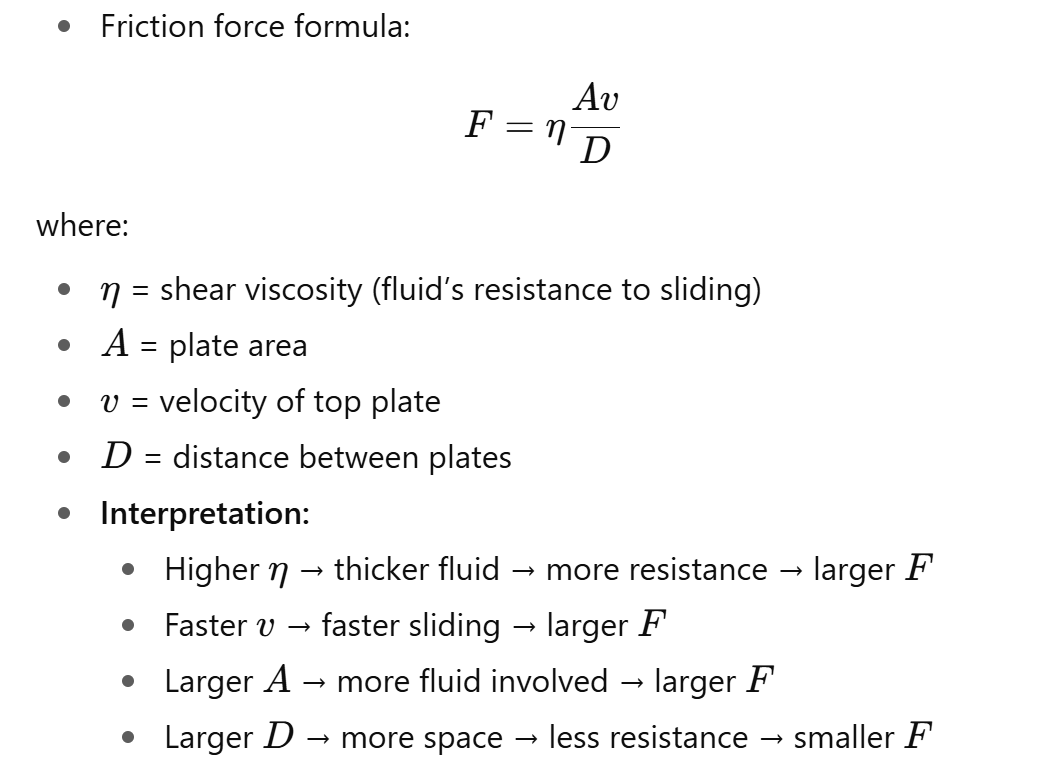
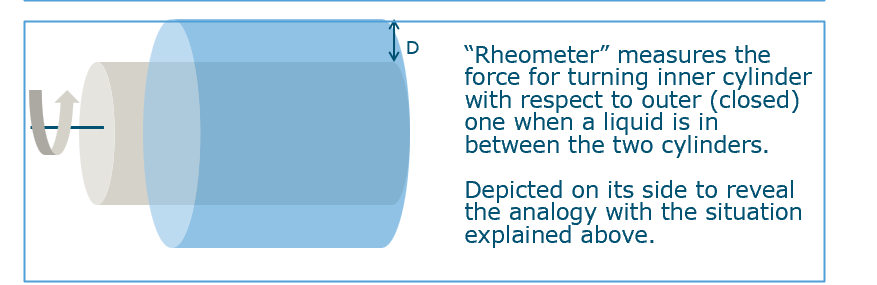
What is the friction force between two parallel plates moving with a fluid in between, and how does it define shear viscosity?
Imagine two plates with fluid between them:
Bottom plate fixed, top plate slides at speed v.
Fluid resists the motion, creating a friction force F.
What is a rheometer and how does it measure viscosity?
Rheometer: apparatus with curved plates forming a closed cylinder containing liquid.
Measures force needed to rotate inner cylinder relative to outer.
Surface area AAA in formula is that of the cylinder.
Allows practical measurement of shear viscosity without liquid running out.
What is the friction force on a sphere moving through a fluid?
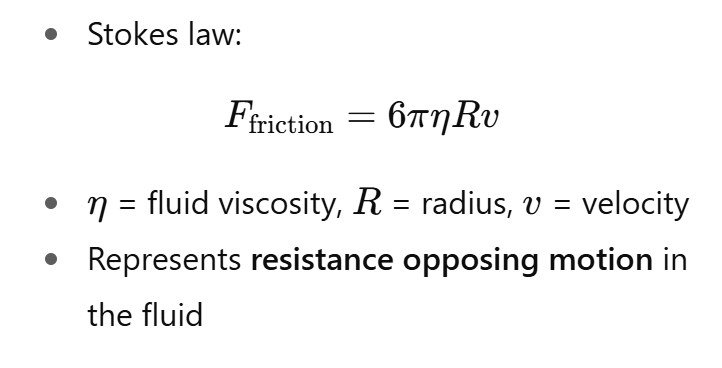
How to calculate a numerical value for creaming velocity?
What is the structural difference between whole milk and homogenised milk regarding fat particles?
Whole milk: large fat globules (~10 µm radius) → cream rises quickly.
Homogenised milk: small fat globules (~1 µm radius) → extremely slow or negligible creaming.
Both: fat globules are the dispersed particles
How does particle size affect creaming time in milk?
Whole milk: radius 10 µm → 20 cm rises in ~20 min
Homogenised milk: radius 1 µm → 20 cm rises in ~2000 min (~33 hours)
Conclusion: smaller particles → slower creaming → more stable milk
Why don’t 1 µm fat particles in homogenised milk cream noticeably?
small fat globules rise so slowly that no visible creaming occurs over practical time scales.
Creaming is caused by the upward buoyant force acting on particles in a fluid, but for particles this small, the force is extremely weak because it scales with the particle’s volume (R3).
At the same time, viscous friction from the fluid strongly resists motion, leaving almost no net force
This means the particles move upward only with a tiny terminal velocity, so small that the fat droplets stay suspended and do not visibly separate within the time scales of storage
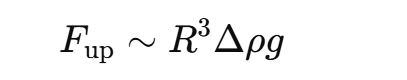
How are macroscopic and molecular physics parameters connected?
Macroscopic: pressure, temperature, volume, viscosity
Molecular: particle size, density, kinetic energy, molecular interactions
External influences: stress/energy, time, temperature, density, molecular composition
What is the units of pV?
(force/area)*volume =force*length = energy
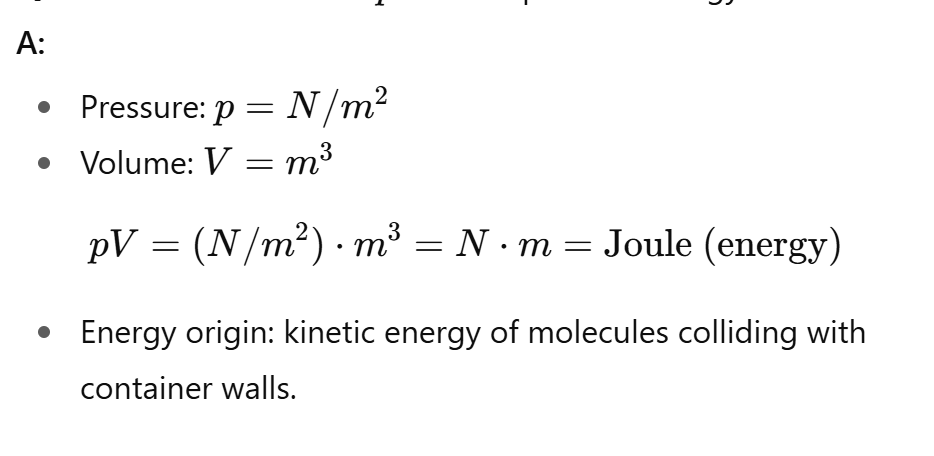
How is the energy of one mole of gas molecules related to the kinetic energy of a single molecule?
The total energy of a mole of gas is simply the sum of the kinetic energies of all the individual molecules.
Each molecule carries about kT of energy, and summing over all molecules in a mole gives the macroscopic energy RT.
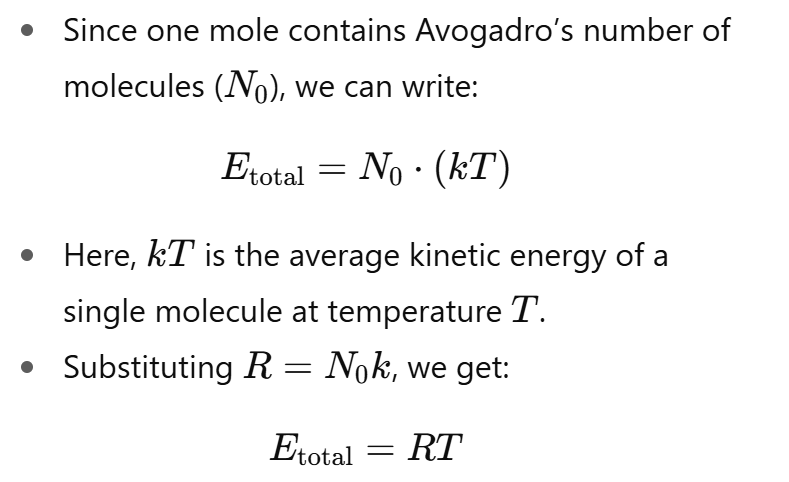
What is the physical meaning of R=N0k
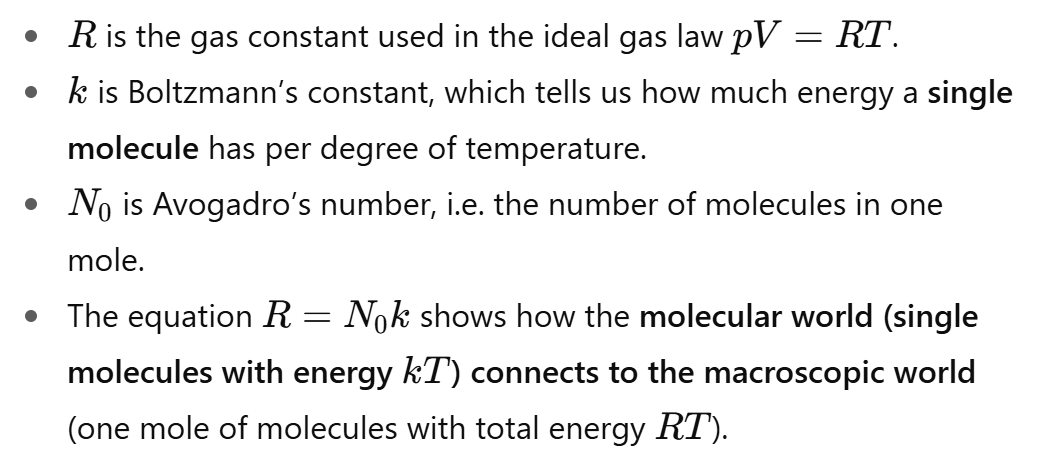
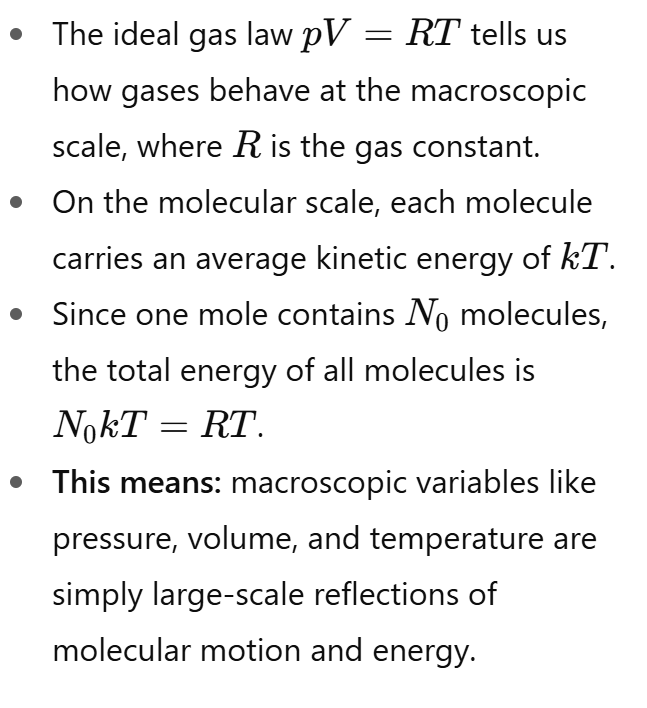
How do macroscopic gas properties connect to molecular scale properties?
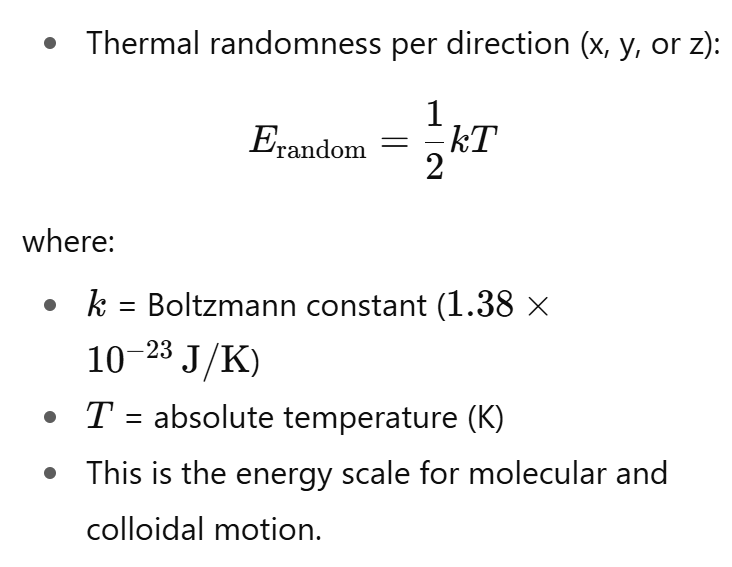
• Particles in a liquid move randomly due to thermal energy, about kT per direction (x, y, z).
• Gravitational motion matters only when the gravitational energy of a particle is larger than this thermal energy.
Egravity > kT
If gravitational energy > kT: creaming
(directed motion dominates).
• If gravitational energy < kT: no
creaming (random motion dominates).
Why do 1 µm particles not cream noticeably?
explain in relation to Brownian motion
For very small particles, the gravitational energy is much smaller than kT.
Brownian motion (random thermal motion) is stronger than the pull of gravity.
As a result, particles keep moving randomly instead of settling or creaming.
Conclusion: For ~1 µm fat globules, thermal energy dominates, preventing creaming.
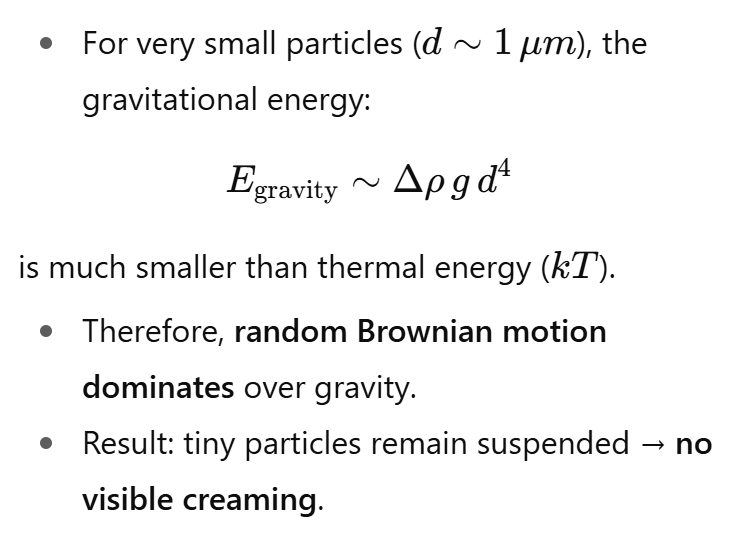
How is gravitational energy expressed for a particle in a fluid?
*pie/6 aswell in the main formula

How is the energy of random motion expressed?
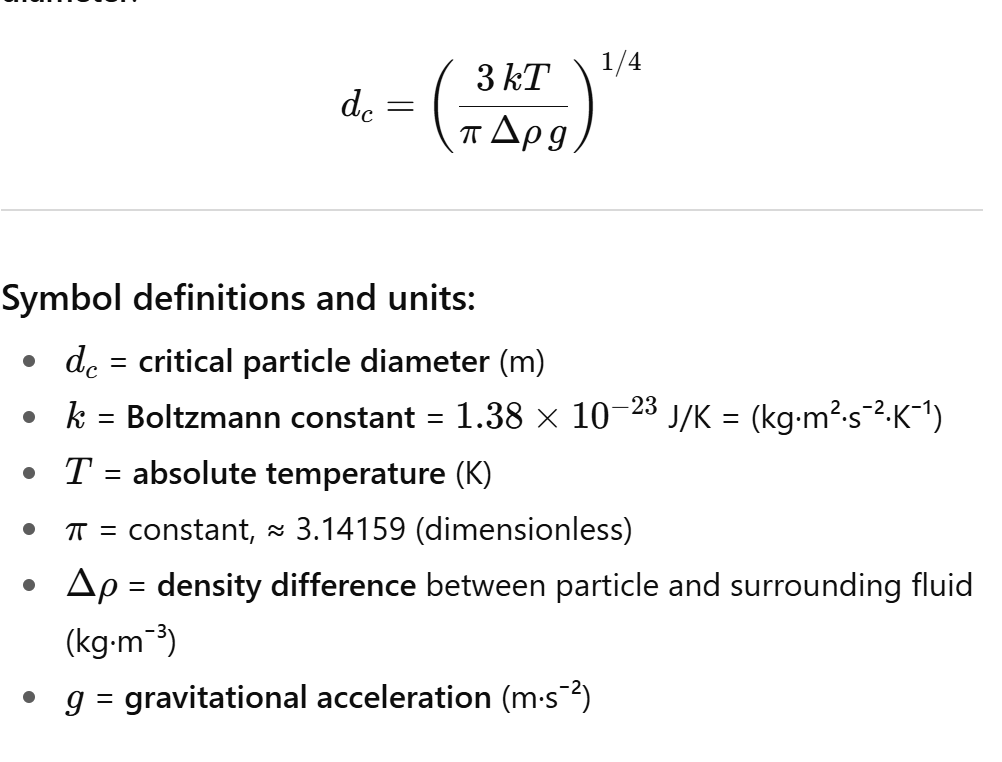
How can we estimate the critical particle size below which no creaming occurs?
Non-creaming criterion (random motion dominates)
Erandom>Egravity
Set equality to find the critical diameter dc where gravity just balances random motion:
What is diffusion and how is it related to randomness of motion?
Even without gravity, molecules move randomly in all directions.
If concentration is higher in region A than region B, more molecules statistically move from A to B than from B to A.
This net movement is called diffusion.
Example: tea or coffee brewing → molecules spread by diffusion, not convection
Why does milk look white?
because fat and protein particles scatter light in all directions (multiple scattering).
Why does the sky look blue, and the sun look red at sunset?
Scattering depends on wavelength: blue light scatters more strongly than red light.
Sky: looks blue because blue light is scattered most by air molecules.
Sun at sunset: looks red because blue light is scattered away, leaving mainly red/orange light.
What is the pH of milk, and what happens upon significant acidification?
Normal milk: pH ≈ 6.5
Upon significant acidification: coagulation occurs
Reason: casein micelles aggregate, forming clumps.
What structural elements give whipped cream its solid-like behaviour?
Air bubbles – occupy most of the space → resist deformation → yield rigidity.
Fat particles – partially coalesced shells stick together → form a network → provide additional rigidity.
Visual suggestion: a schematic showing air bubbles surrounded by fat particles forming a network.
Why does homogenised cream not whip well?
Homogenisation destroys the fat particle shells, preventing partial coalescence.
Fat particles cannot stick together efficiently → weak or no network formation → less rigid whipped cream.
Visual suggestion: compare shell structure of raw fat particle vs homogenised fat particle.
What is the fraction of fat particles in cream versus raw milk, and how does it affect whipping?
Cream: ~40% fat → high chance of fat particles coalescing → network forms → easy whipping.
Raw milk: ~3% fat → lower chance of coalescence → weaker network → harder to whip.
How does viscosity affect whipping and air bubble stability?
Raw milk: low viscosity → air bubbles move easily → liquid flows past bubbles → unstable two-phase system.
Cream: higher viscosity → air bubbles stabilized → network holds → rigid whipped cream.
Visual suggestion: diagram showing air bubbles in low-viscosity milk (bubbles rise/fall) vs high-viscosity cream (bubbles fixed).
Why should cream be used straight from the fridge for whipping?
Fats start to melt at > 7 °C
Molten layers cannot form partially coalesced shells → no network formation → poor whipping.
Cold cream: fat partially solid → forms network → stable whipped cream.
How does fat particle coverage stabilize air bubbles in cream vs milk?
Cream: enough fat particles → air bubbles fully covered → stable network.
Milk: too few fat particles → air bubbles not fully covered → bubbles merge and escape → unstable structure.