Thermochemistry
4.8(6)
Card Sorting
1/27
Earn XP
Description and Tags
Last updated 12:43 AM on 10/27/22
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
28 Terms
1
New cards
What is energy?
The capacity to do work.
2
New cards
What is thermal energy?
The energy associated with the random motion of atoms and molecules.
3
New cards
What is chemical energy?
The energy stored within the bonds of chemical substances.
4
New cards
What is nuclear energy?
The energy stored within the collection of neutrons and protons in the atom.
5
New cards
What is electrical energy?
The energy associated with the flow of electrons.
6
New cards
What is potential energy?
The energy available by virtue of an object’s position.
7
New cards
What is heat?
The transfer of thermal energy between two bodies that are at different temperatures.
8
New cards
What is temperature?
The measure of thermal energy
9
New cards
What is different about temperature and thermal energy?
They are different because temperature is the measure of thermal energy.
10
New cards
What is thermochemistry?
The study of heat change in chemical reactions.
11
New cards
What is a system? What is around the system?
The specific part of the universe that is of interest in the study (in this case, it is the chemical reaction). Everything around the system is the surroundings.
12
New cards
Why would a bathtub of 40°C have more thermal energy than a mug of 90°C?
It has more thermal energy because of its mass. The amount of electrons and heat in the tub is greater than the mug.
13
New cards
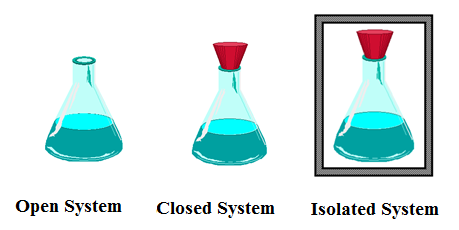
Types of systems
Open, closed, and isolated.
14
New cards
What is exchanged in a open system?
Mass and energy
15
New cards
What is exchanged in a closed system?
Energy
16
New cards
What is exchanged in an isolated system?
Nothing
17
New cards
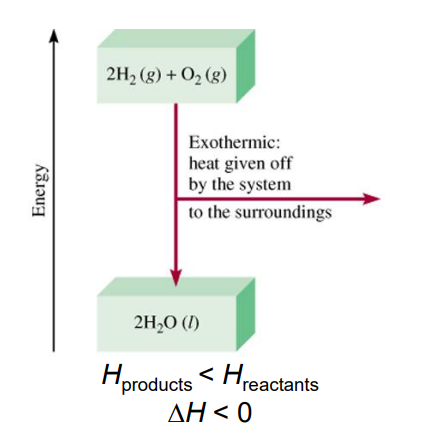
What is an exothermic process
Any process where heat is given off. Transfers thermal energy from system to surroundings.
18
New cards
What is a thermochemical equation
A balanced chemical equation plus an energy term.
19
New cards
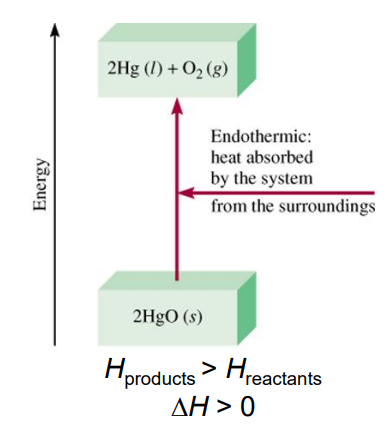
What is an endothermic process?
Any process where heat is supplied to system from surroundings. Heat is applied to reaction.
20
New cards
Exothermic process equation
heat is a product
21
New cards
Endothermic process equation
heat is a reactant
22
New cards
What is enthalpy (H)
Enthalpy is used to quantify heat flow into or out of a system in a process that occurs at a constant pressure.
23
New cards
Change in heat equation
ΔH = H(products) - H(reactants)
24
New cards
ΔH = ?
= heat given off/absorbed during chemical reaction
25
New cards
Negative sign
Exothermic reaction
26
New cards
Positive sign
Endothermic reaction
27
New cards
What do stoichiometric coefficients refer to?
The number of moles in a substance
28
New cards
Reverse reactions means...
The sign of ΔH changes