BC.06 Double bonds, configurations and conformations
0.0(0)
Card Sorting
1/11
Earn XP
Description and Tags
Last updated 4:40 PM on 1/9/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
12 Terms
1
New cards
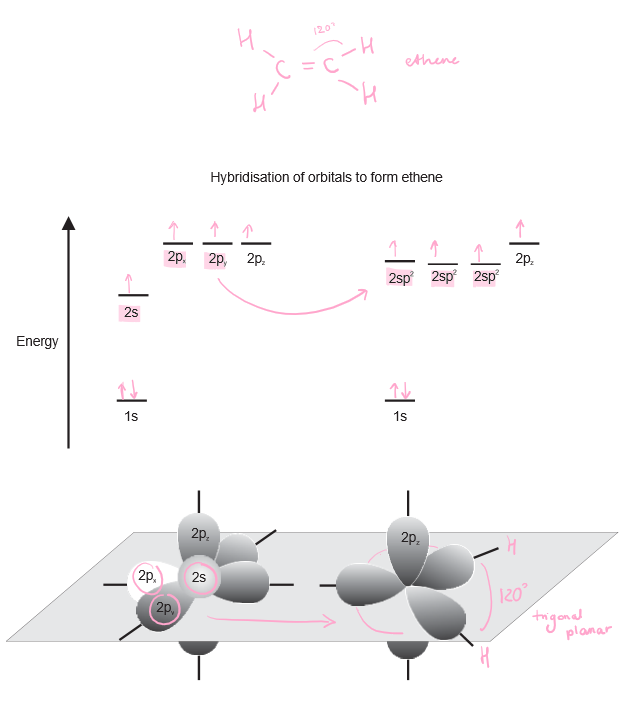
Detail how and when sp² hybridisation occurs.
In for example ethene molecules, sp² hybridisation occurs to create a 120 degree trigonal planar shape. 1 2s orbital and 2 2p orbitals combine to form 2 sp² hybrid orbitals.
2
New cards
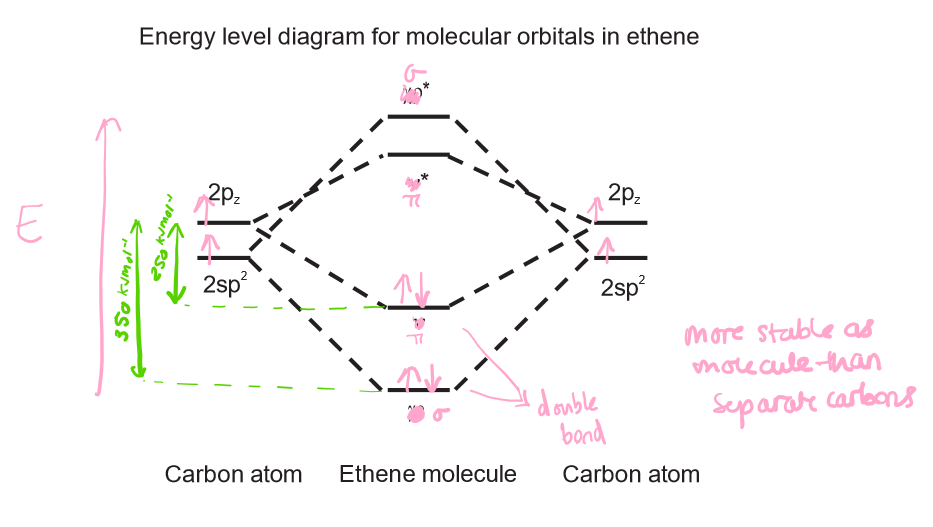
Draw an energy level diagram for molecular orbitals in ethene.
3
New cards
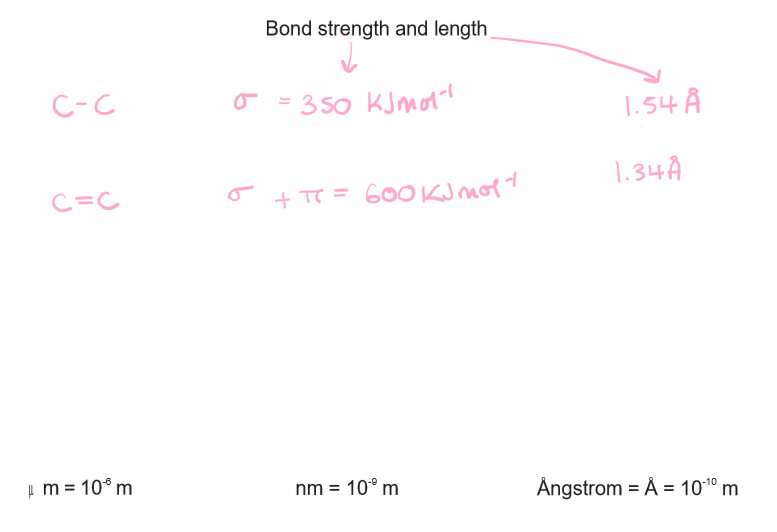
What is the bond strength and length of a sigma bond and a pi bond?
4
New cards
Draw the cis and trans configurations of an ethene molecule
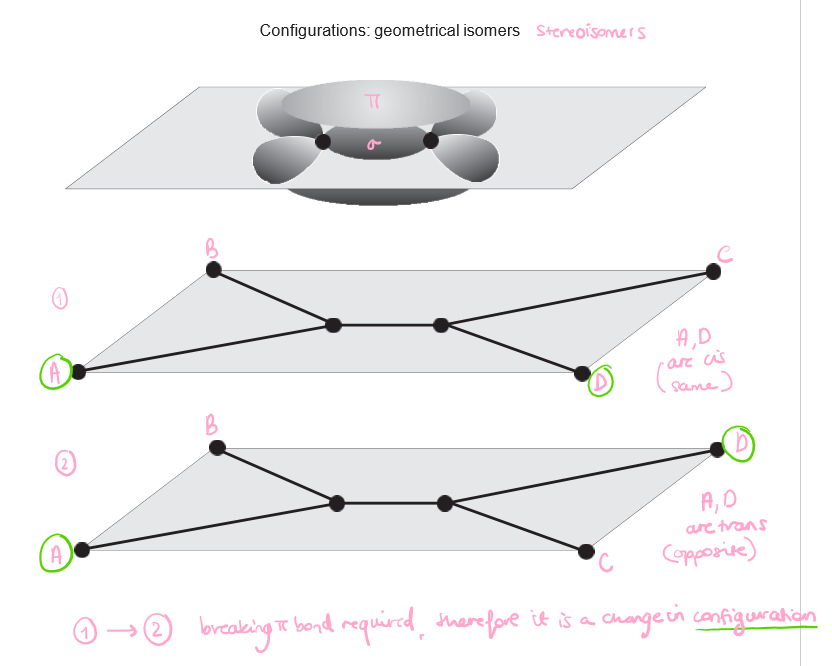
5
New cards
What are conformations?
Rotations around single bonds
6
New cards
Illustrate eclipsed and staggered conformations of an ethene molecule.
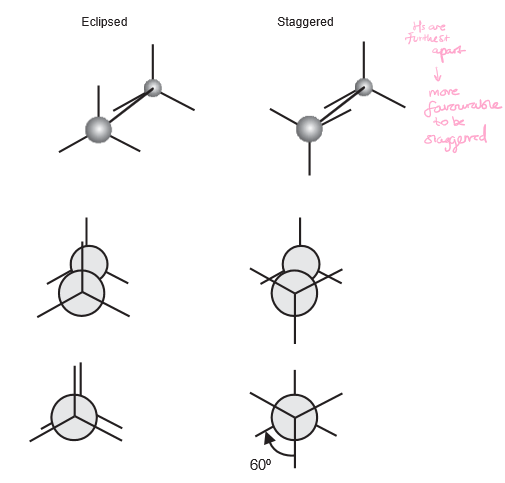
7
New cards
What is the energy cost for changing conformations (rotating bonds) between staggered and eclipsed?
10 kJ/mole
8
New cards
What are the different staggered conformations?
\
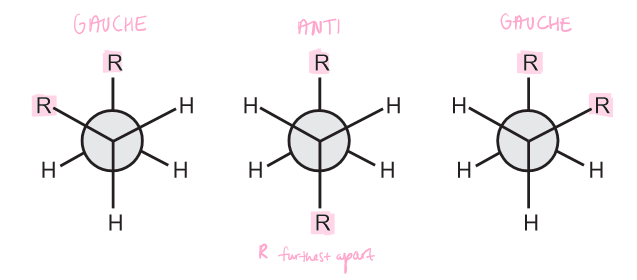
9
New cards
What is the energy cost to rotate the bond to create the different staggered conformations?
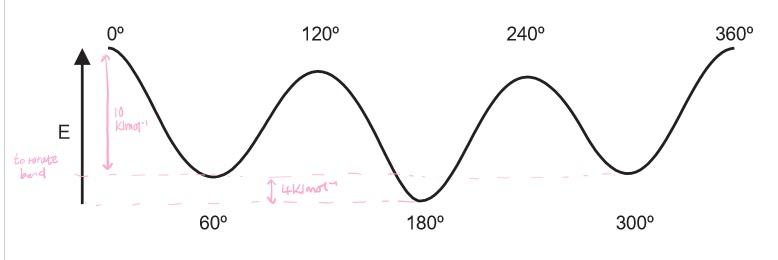
10
New cards
Draw the 4 conformations (2 bonding orbitals, 2 anti-bonding orbitals) of a 1,3-butadiene molecule, showing the nodes.
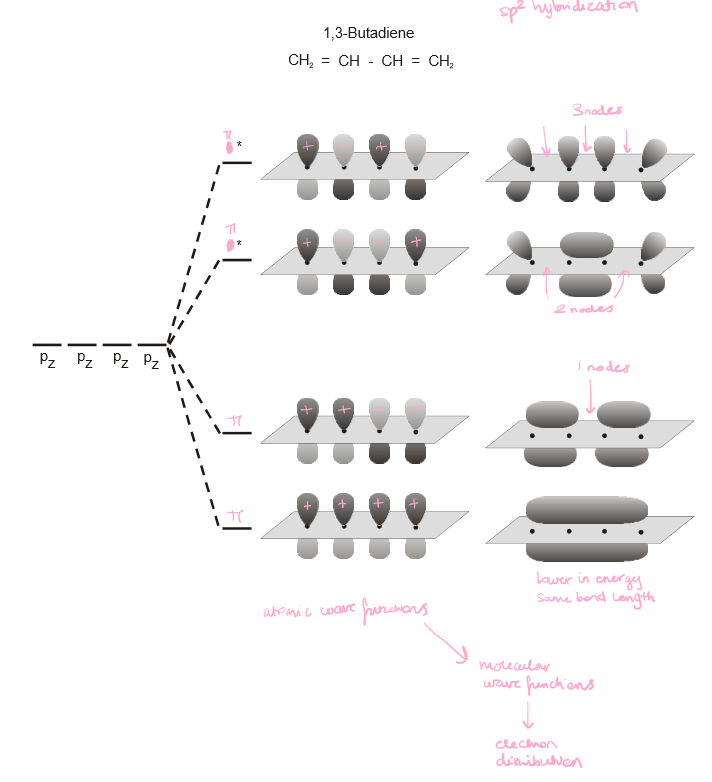
11
New cards
Benzene, an sp² hybridised aromatic molecule, has at least 6 conformations and all 6 electrons of the delocalised pi system. Draw 6 conformations of benzene.
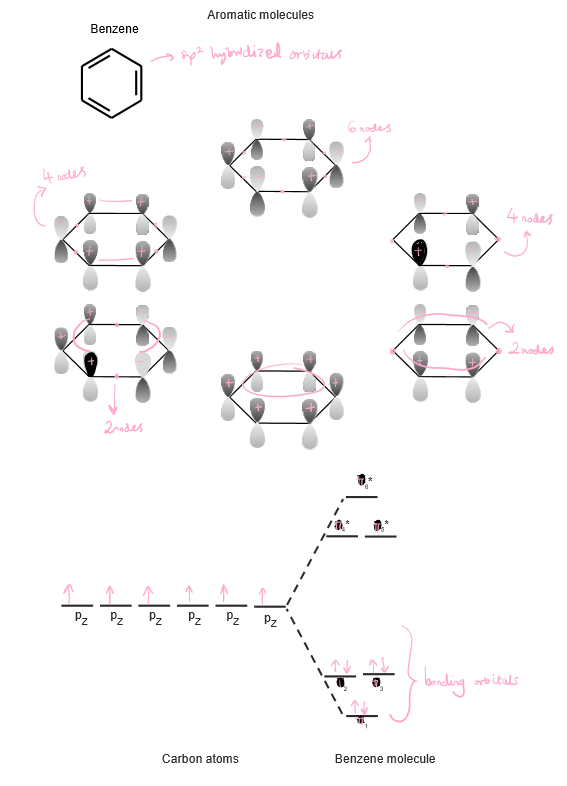
12
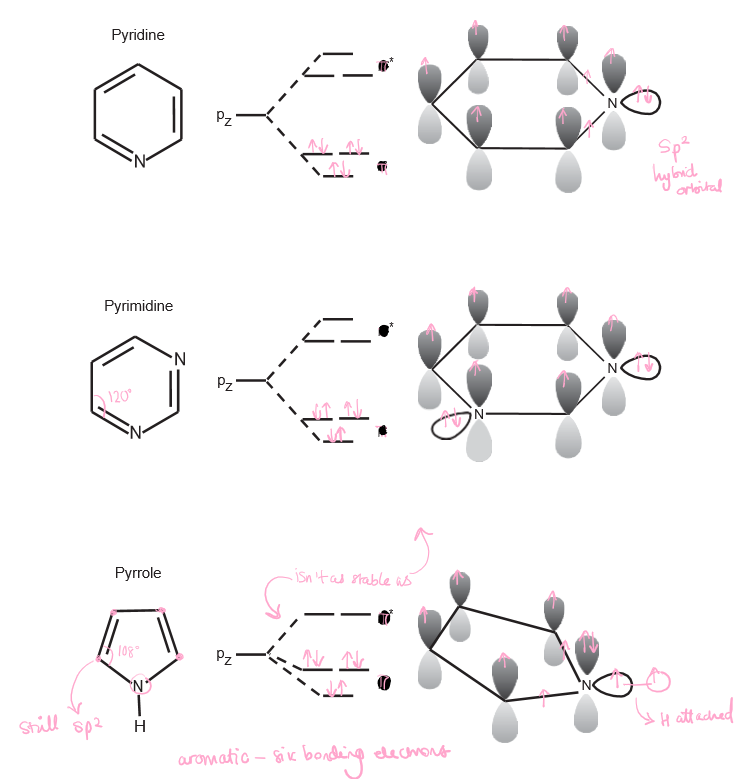
New cards
Pyridine and pyrimidine are all heterocyclic aromatic molecules that are sp² hybridised. Draw the electron orbitals of pyrrole, decide whether is it sp² hybridised and detail how stable this molecule is in comparison to pyrimidine/pyridine.