Food and Energy Practical
1/20
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
21 Terms
Energy content from food
Different foods have different energy contents, some act as quick sources of energy and others act as long-term stores of energy, such as fat

Balancing energy
The energy content of food eaten must be balanced with energy needs since excess energy will be stored as fat by the body, leading to obesity
Energy and food content requirements
Vary between individuals and depend on factors such as age, gender and activity levels

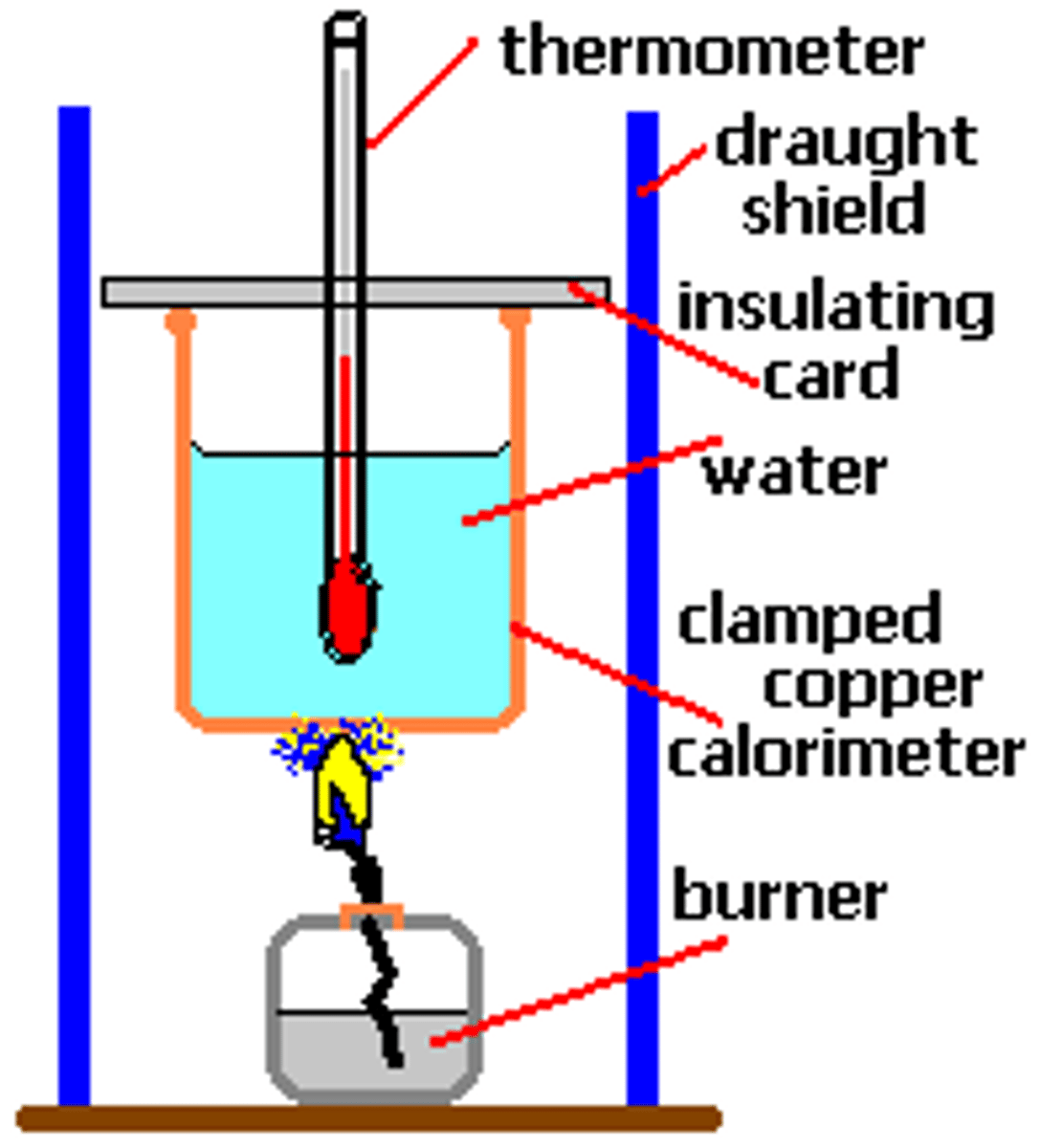
Calorimeter
An instrument used to measure changes in thermal energy
Burning food
Can be used to demonstrate the energy content of different food items
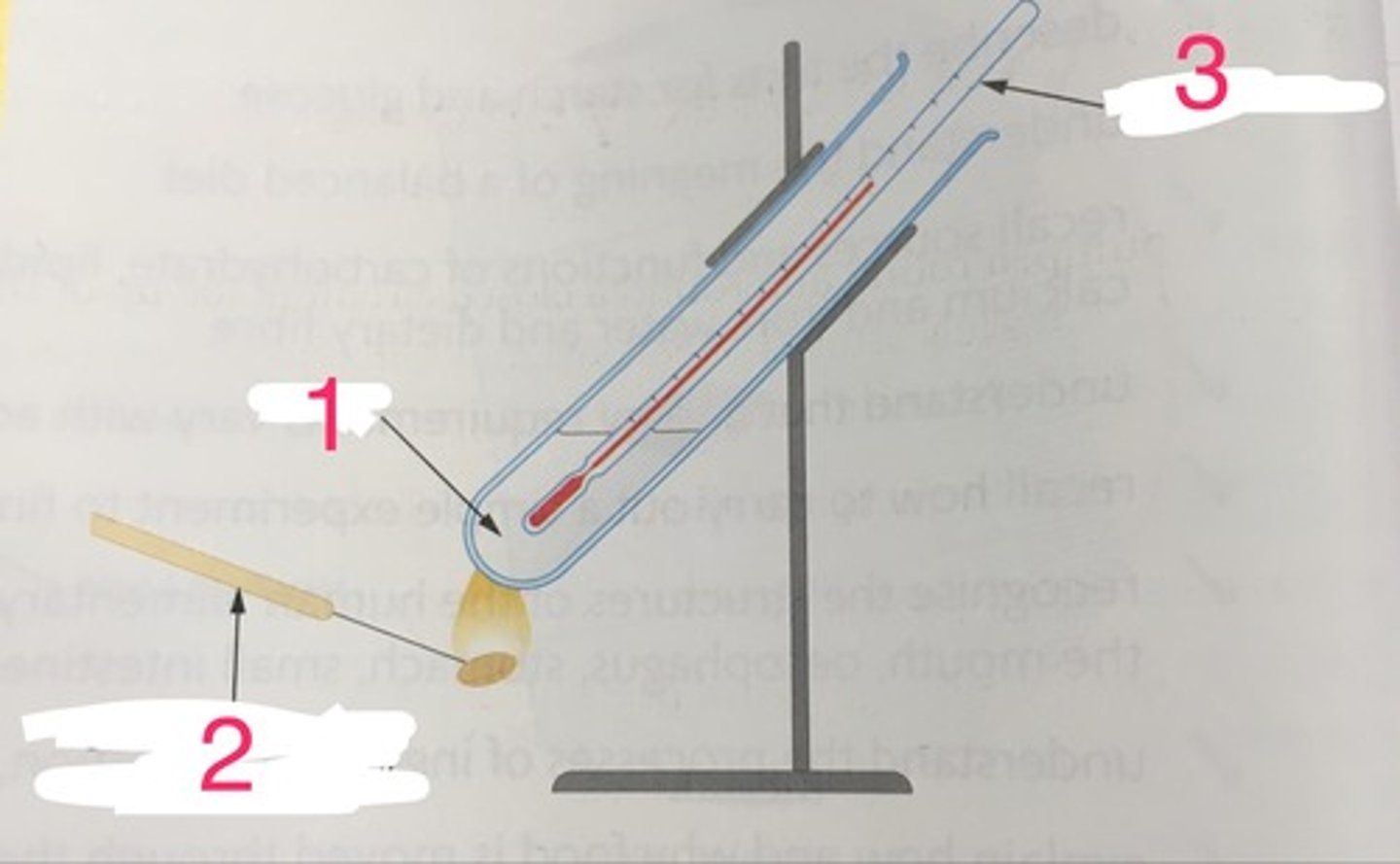
Mounted needle
A needle mounted in a wooden or metal handle that is used to impale a food source and hold it over a bunsen burner

Thermometer
An instrument used to measure temperature
Bunsen burner
Used safely to heat food products until they set alight, after which the food product is suspended below a boiling tube containing water and a thermometer

Boiling tube
Holds a set volume of water and a thermometer to act as a calorimeter

Clamp, boss and clamp stand
Keeps the boiling tube and water suspended at an appropriate height and angle

Electronic balance
A piece of equipment used to measure the mass of each food source

Dependent variable
The variable that is measured, in this case it is the energy content released from food or the change in temperature that results from burning food
Independent variable
The variable that is changed, in this case it is the type of food source that is burned

Control variables
Variables that are kept the same in an experiment such as the volume of water in the boiling tube, size or quantity of food sample used, distance of burning food source from boiling tube
Acclimatisation
It is important that the boiling tube with water is allowed to return to room temperature or a fresh water sample can be used between tests
Energy and temperature
The energy content of food can be determined by burning a food sample and immediately suspending it under a boiling tube of water with a thermometer in it, the temperature change suggests the energy content

Burning food process
Light a sample of food using a bunsen burner, move the burning food sample away from the bunsen burner and place it under a calorimeter, measure the temperature change that results from the burning food
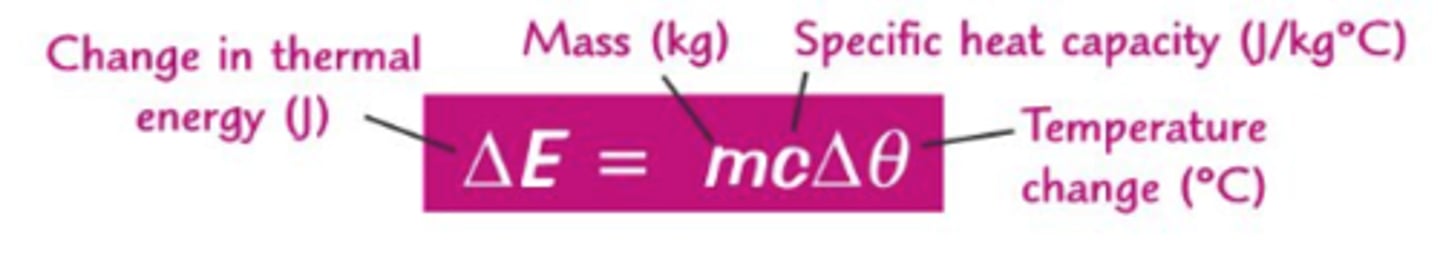
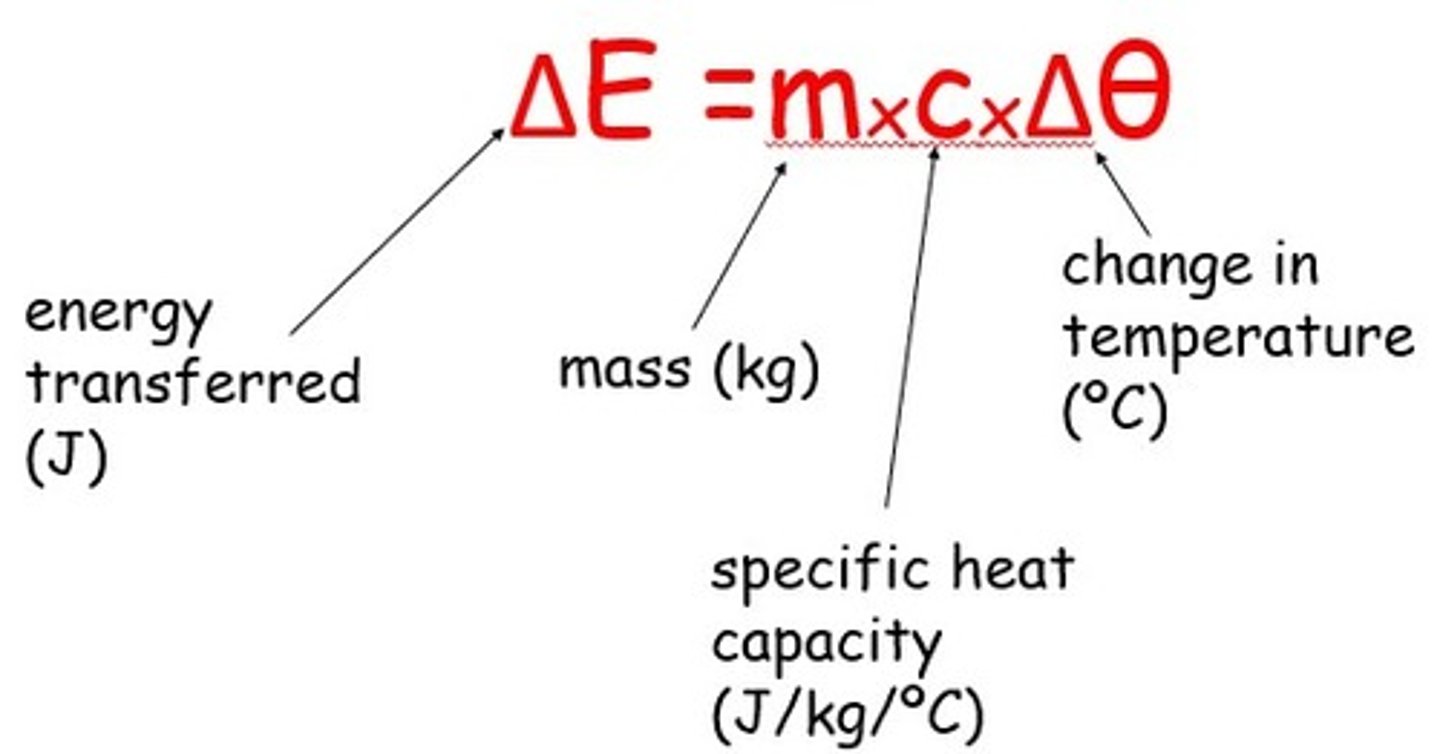
Energy released from food equation
Energy released from food per gram = (mass of water x temperature increase x 4.2)/mass of food sample
Units for energy, mass, temperature
Energy is measured in Joules, mass for this experiment is measured in (kilo)grams, temperature is measured in degrees Celsius
One gram
The mass of one cm^3 of water
Specific heat capacity
The energy required to raise a unit mass of a substance by one degree Celsius, for water this value is 4.2 Joules per gram per degree Celsius