Ionic Crystal Structures (copy)
0.0(0)
Card Sorting
1/8
Earn XP
Description and Tags
Last updated 10:15 PM on 12/8/22
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
9 Terms
1
New cards
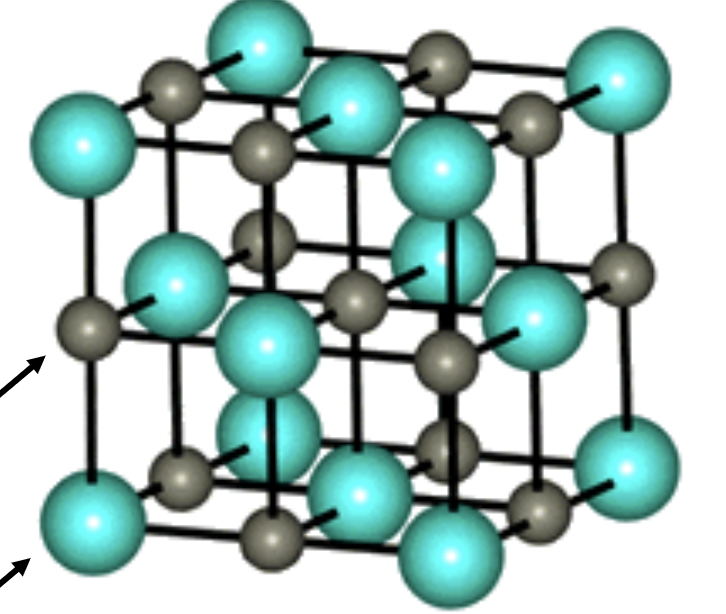
Rock Salt
1:1 stoichiometry
A and X are 6 coordinate
face centered cubic lattice
octahedral holes
2
New cards
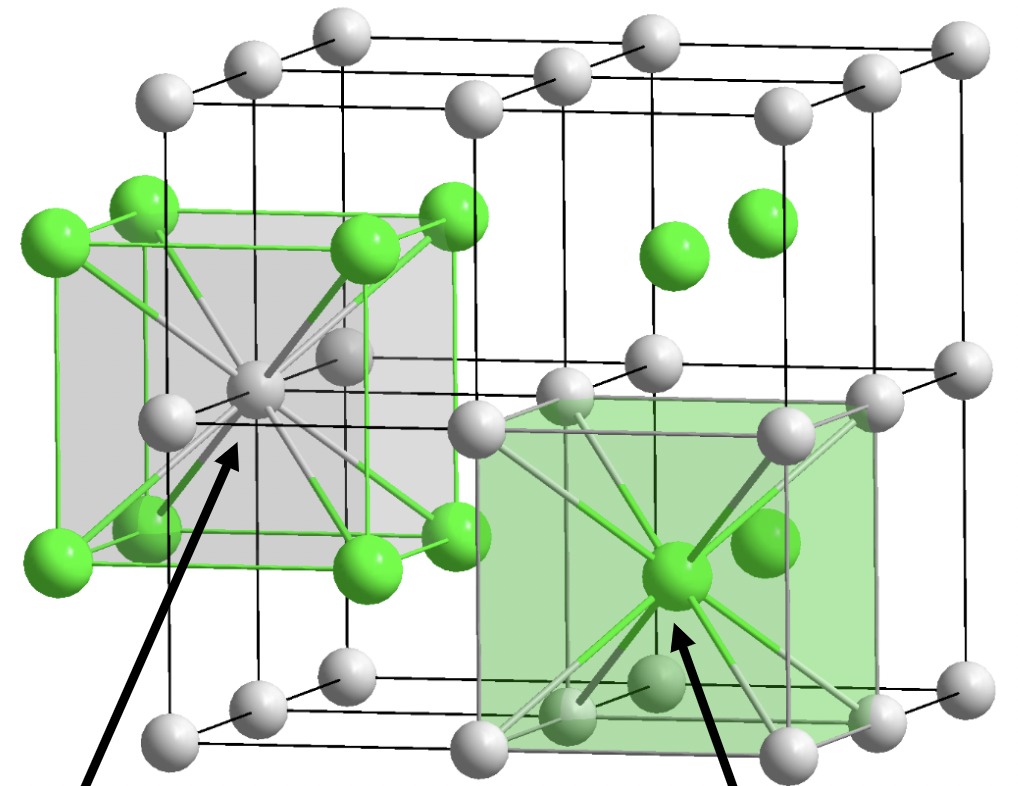
CsCl
1:1 stoichiometry
A and X are 8 coordinate
primitive cubic lattice
octahedral holes
often large cation with small anion
3
New cards
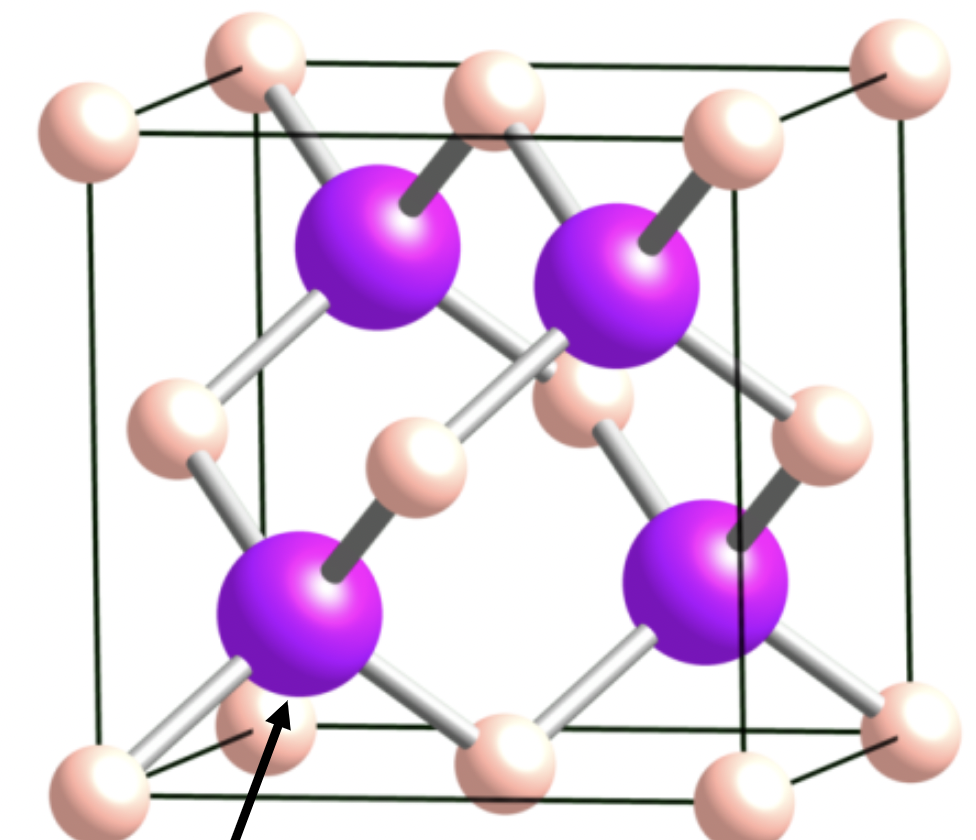
Zinc Blende
1:1 stoichiometry
A and X are 4 coordinate
FCC lattice
tetrahedral holes
important for semi conductors
looks like diamond but with mix of ions
A and X are 4 coordinate
FCC lattice
tetrahedral holes
important for semi conductors
looks like diamond but with mix of ions
4
New cards
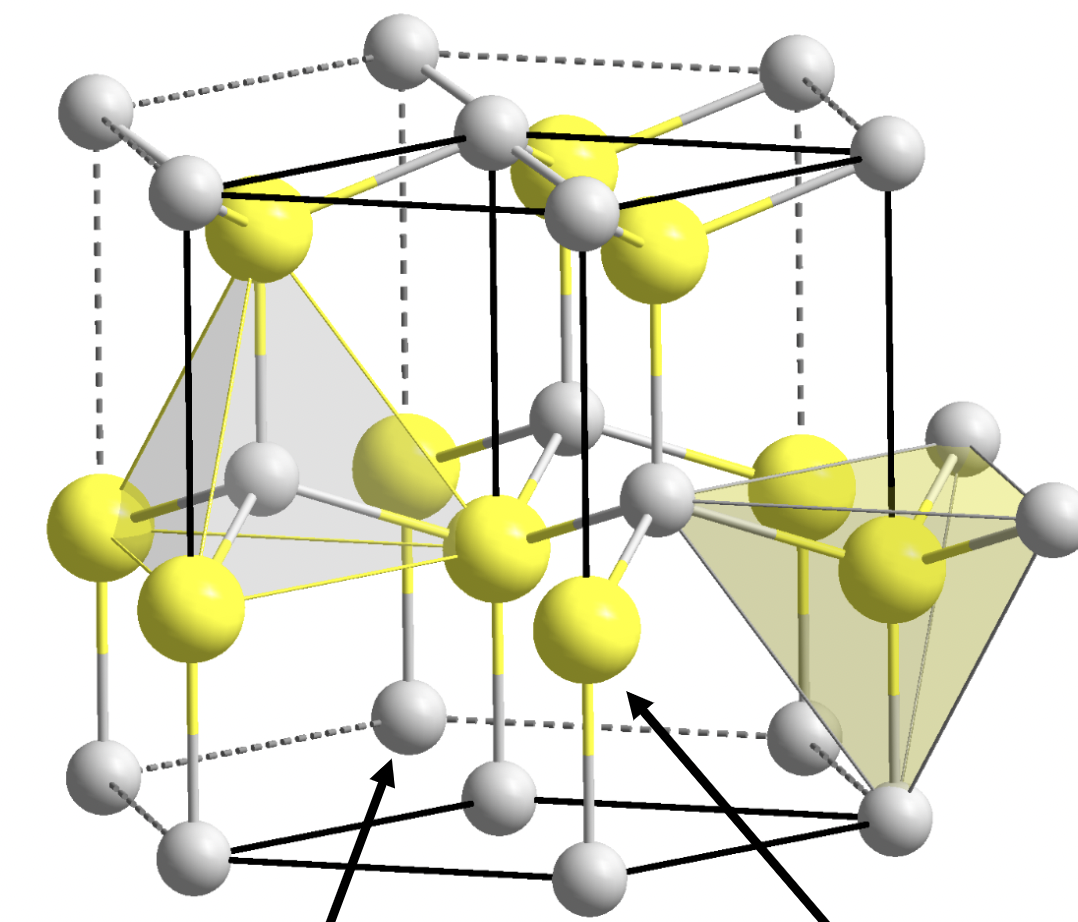
Wurtzite
1:1 stoichiometry
A and X are 4 coordinate
hexagonal close packed lattice
tetrahedral holes
A and X are 4 coordinate
hexagonal close packed lattice
tetrahedral holes
5
New cards
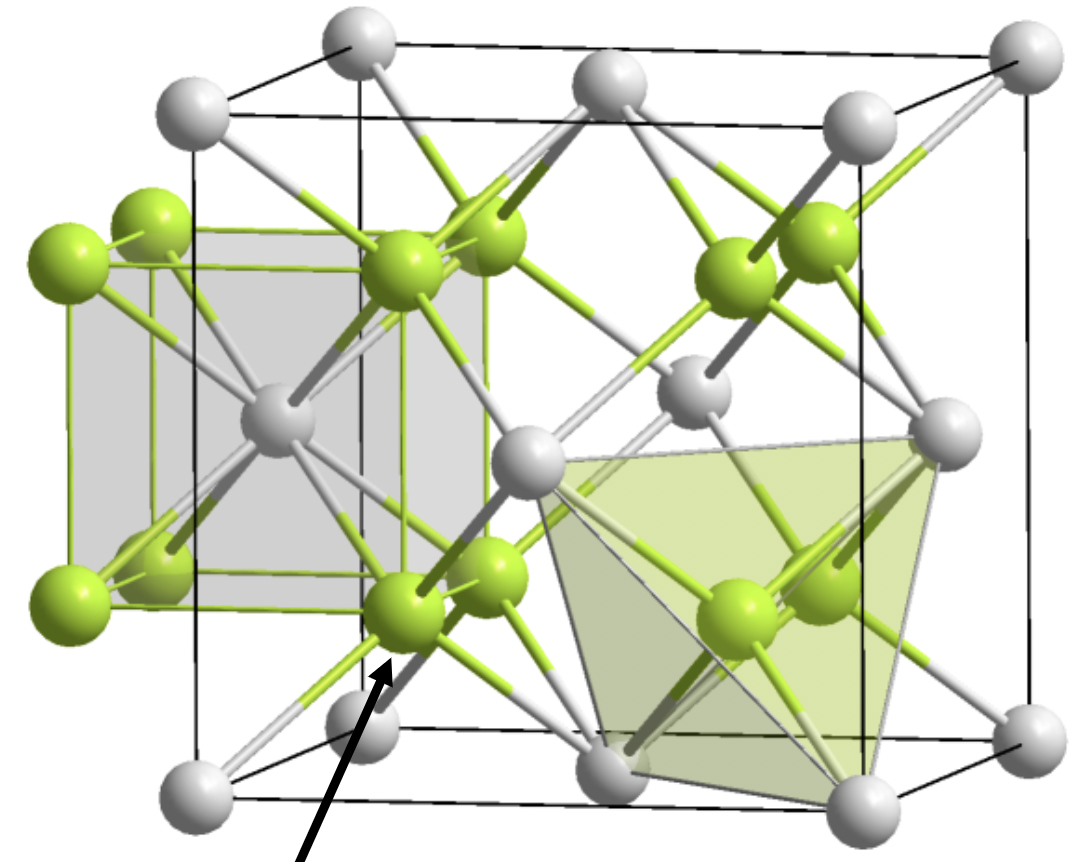
Fluorite
Corners- cation
1:2 stoichiometry
A is 8 coordinate, X is 4 coordinate
FCC lattice
X in tetrahedral holes of A lattice
common compounds: halides + alkali earth metals
1:2 stoichiometry
A is 8 coordinate, X is 4 coordinate
FCC lattice
X in tetrahedral holes of A lattice
common compounds: halides + alkali earth metals
6
New cards
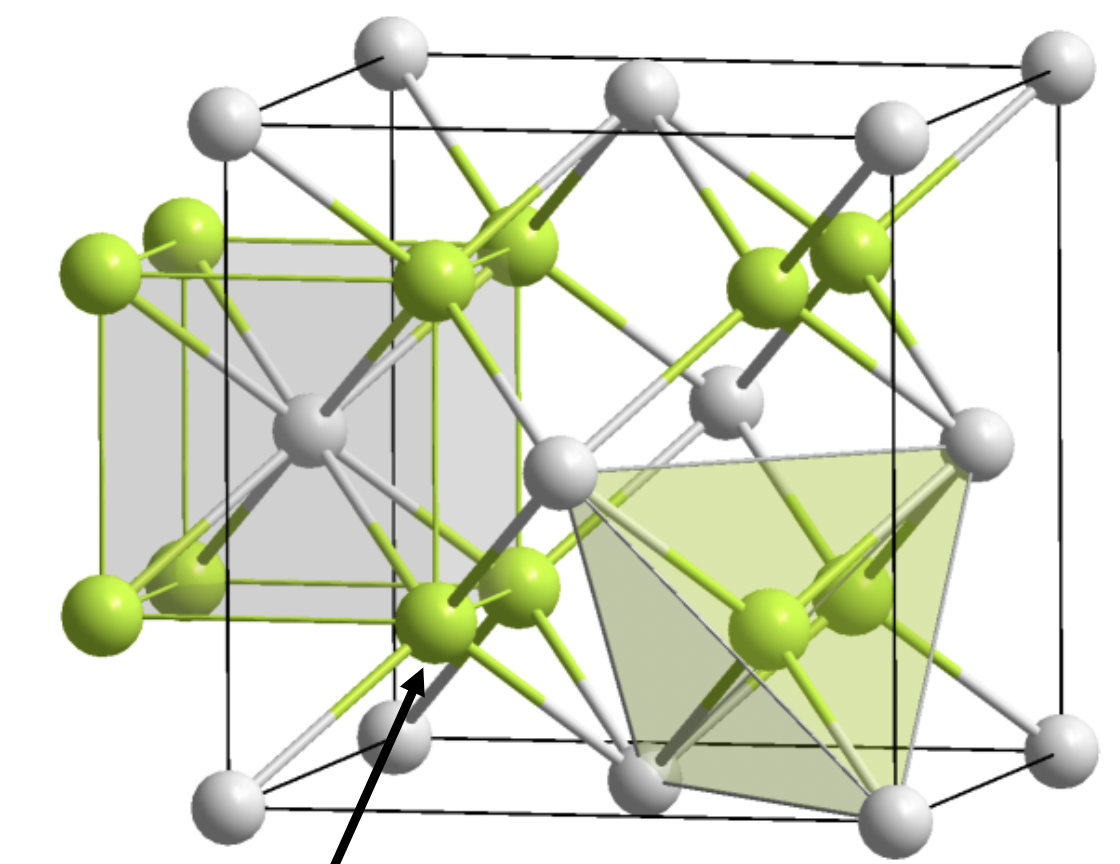
Anti-fluorite
Corners- anion
2:1 stoichiometry
A is 4 coordinate, X is 8 coordinate
FCC latice
A in tetrahedral holes of X lattice
common compounds: oxides (Li2O, Na2O, etc)
2:1 stoichiometry
A is 4 coordinate, X is 8 coordinate
FCC latice
A in tetrahedral holes of X lattice
common compounds: oxides (Li2O, Na2O, etc)
7
New cards
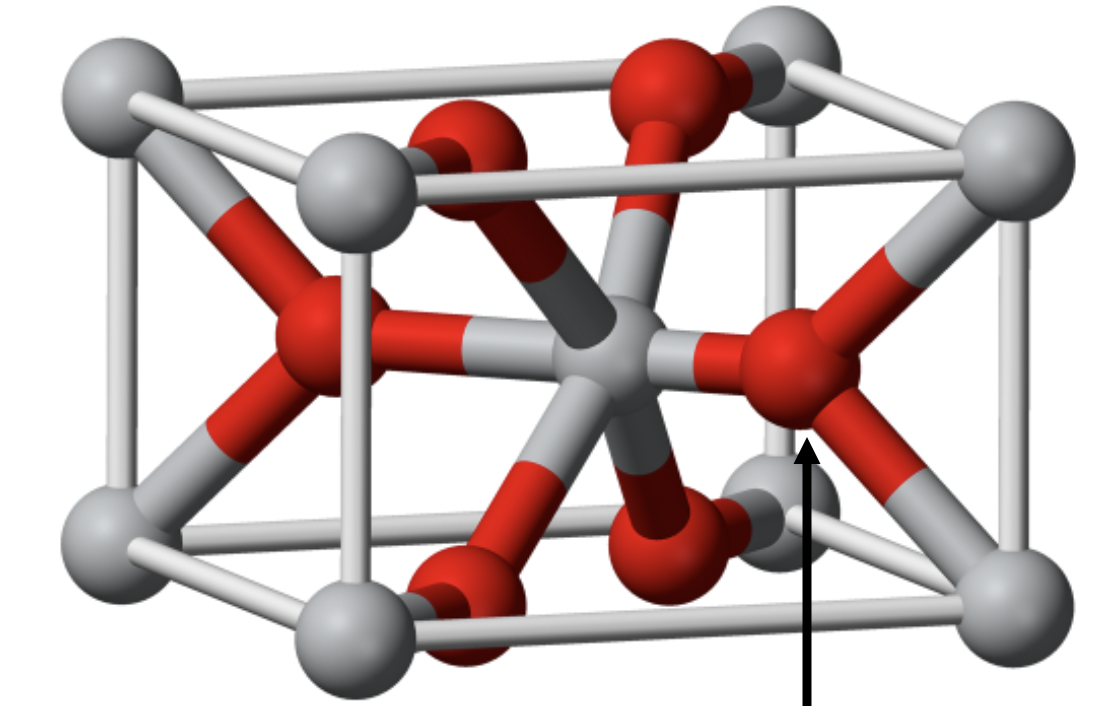
Rutile
1:2 stoichiometry
A is 6 coordinate, X is 3 coordinate
body centered tetragonal
A is 6 coordinate, X is 3 coordinate
body centered tetragonal
8
New cards
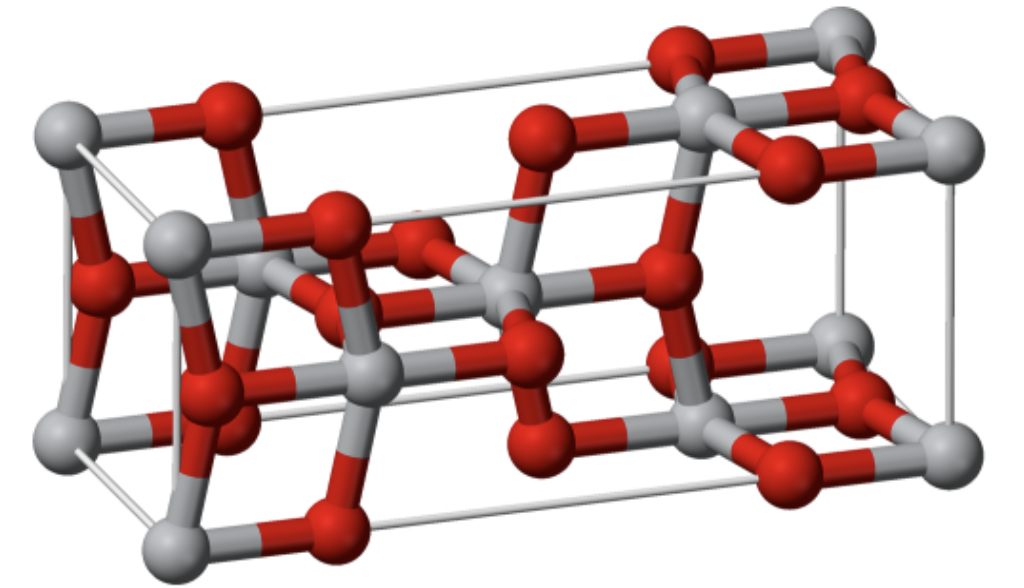
Anatase
polymorph of rutile
9
New cards
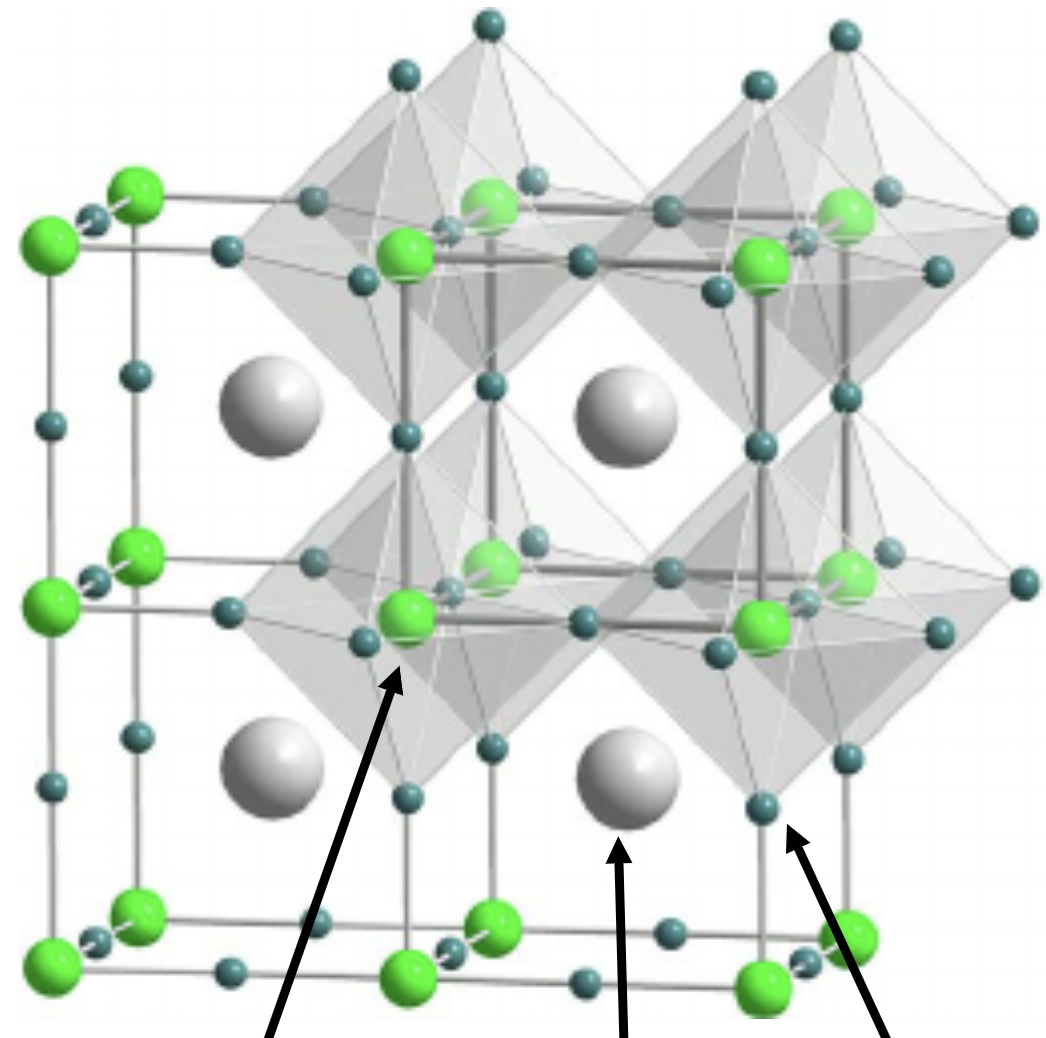
Perovskite
1:1:3 stoichiometry
A is 12 coordinate, B is 6 coordinate
X is 6 coordinate (4 with A, 2 with B)
Primitive cubic lattice
A in central octahedral hole w/in B lattice
A cation has a lower oxidation state than B cation
A is 12 coordinate, B is 6 coordinate
X is 6 coordinate (4 with A, 2 with B)
Primitive cubic lattice
A in central octahedral hole w/in B lattice
A cation has a lower oxidation state than B cation