Alicylic Chemistry-Short Answer Flashcards-
1/85
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
86 Terms
What is alicyclic chemistry?
Answer:Alicyclic chemistry is the chemistry of ring-shaped compounds.
What are cycloalkanes also known as?
Answer:Cycloalkanes are also known as alicyclic compounds.
What is the general formula for cycloalkanes?
Answer:CnH2n
Why do cycloalkanes have fewer hydrogen atoms than acyclic alkanes?
Answer: Because the ring structure requires two fewer hydrogen atoms than the corresponding open-chain alkane.
How are simple cycloalkanes named?
Answer: By adding the prefix "cyclo" to the name of the corresponding alkane.
How are cycloalkane structures typically shown in diagrams?
Answer: As regular polygons with the number of vertices equal to the number of carbon atoms.
What determines whether a compound is named as an alkyl-substituted cycloalkane or a cycloalkane-substituted alkane?
Answer:The portion (cyclic or acyclic) containing more carbon atoms determines the base name.
When naming a compound with both cyclic and acyclic portions, what is Rule 1 for determining the base name?
Answer: Decide whether the cyclic or acyclic portion contains more carbons; this determines the base name.
How are carbons numbered in a cycloalkane?
Answer: Carbons are numbered to give the lowest numbers for substituted carbons.
When does a cyclic portion become a cycloalkyl substituent?
Answer: When there are more acyclic than cyclic carbons.
True or False: When numbering a cycloalkane with substituents, you always start at a substituted carbon atom.
Answer: True
When numbering a cycloalkane with multiple substituents, which rule determines where to start numbering?
Answer: Numbering starts at the most substituted carbon and proceeds to give the lowest numbers for substituted carbons.
How do boiling and melting points of cycloalkanes compare to those of comparable alkanes?
Answer: Cycloalkanes have higher boiling and melting points than comparable alkanes.
Why do cycloalkanes have higher boiling points than comparable alkanes?
Answer: Their rigid structure permits greater attractive interactions.
What causes cycloalkanes to have higher density compared to corresponding alkanes?
Answer: Their rigid structure allows molecules to "pack" more effectively, increasing mass per unit volume.
How does molecular mass affect the physical properties of cycloalkanes?
Answer: Boiling and melting points increase with increasing molecular mass.
Why can cycloalkanes exhibit geometric isomerism while open-chain alkanes cannot?
Answer: Cycloalkanes cannot undergo free rotation about their C-C bonds due to their ring structure.
What determines whether a disubstituted cycloalkane is a cis or trans isomer?
Answer: The relative position of the substituents on the ring (same side vs. opposite sides).
Define cis isomer in the context of cycloalkanes.
Answer: When two non-hydrogen substituents are on the same side of the ring.
Define trans isomer in the context of cycloalkanes.
Answer: When two non-hydrogen substituents are on opposite sides of the ring.
True or False: A monosubstituted cycloalkane can exhibit geometric isomerism.
Answer: False
Who postulated the theory of angle strain for cycloalkanes?
Answer: Baeyer (1885)
What is the ideal tetrahedral bond angle for sp³ hybridized carbon?
Answer: 109.5°
What is angle strain (Baeyer strain)?
Answer: The strain resulting from deviation of bond angles from the ideal tetrahedral angle of 109.5°.
Why does cyclopropane experience significant angle strain?
Answer: Its bond angles are 60°, which is a significant deviation from the ideal tetrahedral angle of 109.5°.
Where does Baeyer's theory fail?
Answer: It fails for rings with more than four carbon atoms because it assumes all cycloalkanes are planar.
Why does Baeyer's theory not apply well to rings with more than four carbon atoms?
Answer: Because larger cycloalkanes adopt puckered three-dimensional conformations that allow bond angles to be nearly tetrahedral.
What is the relationship between ring size and stability according to experimental data?
Answer: Cyclohexane is the most stable, while cyclopropane and cyclobutane are highly strained.
Name the three types of strain that can affect cycloalkanes.
Answer: Angle strain, torsional/bond strain, and steric strain.
Define angle strain.
Answer: Strain resulting from expansion or compression of bond angles away from their most stable value.
What is meant by "ring strain"?
Answer: The combination of angle strain and torsional strain in cyclic compounds.
Define torsional/bond strain.
Answer: Strain due to the eclipsing of bonds on neighboring atoms.
Define steric strain.
Answer: Repulsive interactions between non-bonded atoms in close proximity.
How is ring strain calculated experimentally?
Answer: Using heats of combustion measurements.
Which cycloalkane has the highest total ring strain?
Answer: Cyclopropane (27.6 kcal)
Which cycloalkane has zero ring strain?
Answer: Cyclohexane
What is the total ring strain in cyclopropane in kcal?
Answer: 27.6 kcal
True or False: Cyclopentane has more ring strain than cycloheptane.
Answer: False (Cyclopentane has 1.3 kcal/mol while cycloheptane has 0.9 kcal/mol per CH₂)
How does the heat of combustion per CH₂ relate to ring strain?
Answer: Higher heat of combustion per CH₂ indicates higher ring strain.
What are the two main factors contributing to cyclopropane's high ring strain?
Answer: Angle strain and torsional strain.
Why are bonds in cyclopropane considered "bent"?
Answer: The enforced 60° bond angle leads to poor overlap of the sp³ orbitals.
What is the bond angle in cyclopropane?
Answer: 60°
Is cyclobutane perfectly planar? Why or why not?
Answer: No, because it adopts a slightly puckered conformation to reduce torsional strain.
What is the approximate bond angle in cyclobutane?
Answer: 88°
What conformation does cyclopentane adopt and why?
Answer: It adopts a puckered "envelope" conformation to reduce torsional strain.
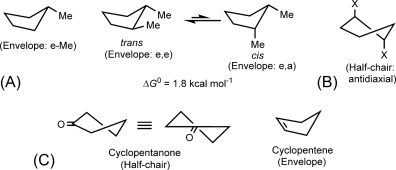
Describe the "envelope" conformation of cyclopentane.
Answer: Four carbons are in the same plane while the fifth carbon is twisted out of plane.
Why is cyclohexane the most common cycloalkane in nature?
Answer: Because it can adopt conformations with very little or zero strain.
What are the two main conformations of cyclohexane?
Answer: Chair and boat conformations.
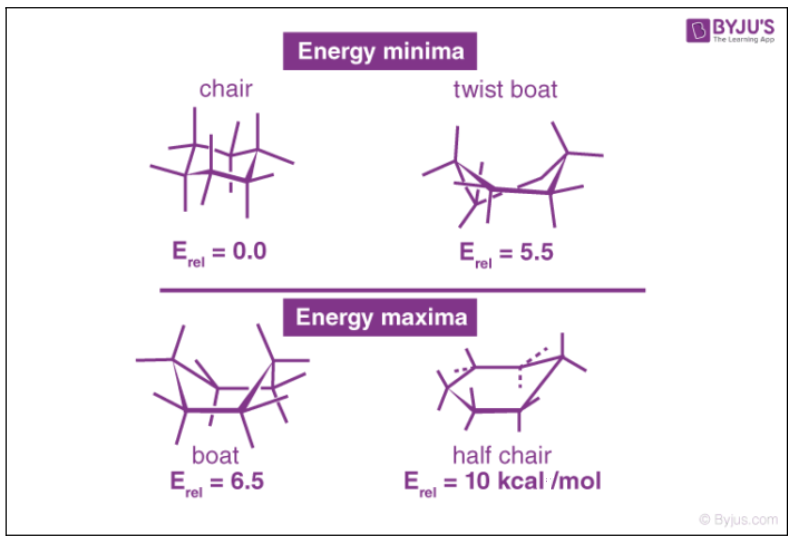
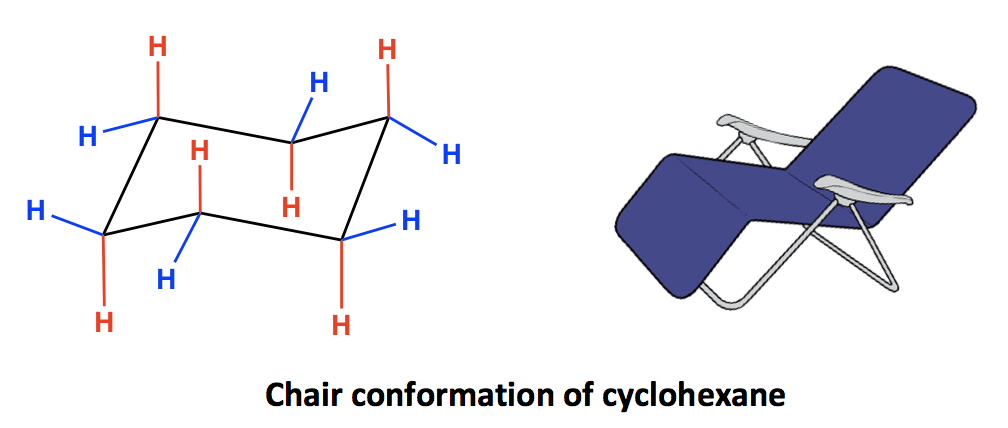
What is the most stable conformation of cyclohexane?
Answer: The chair conformation.
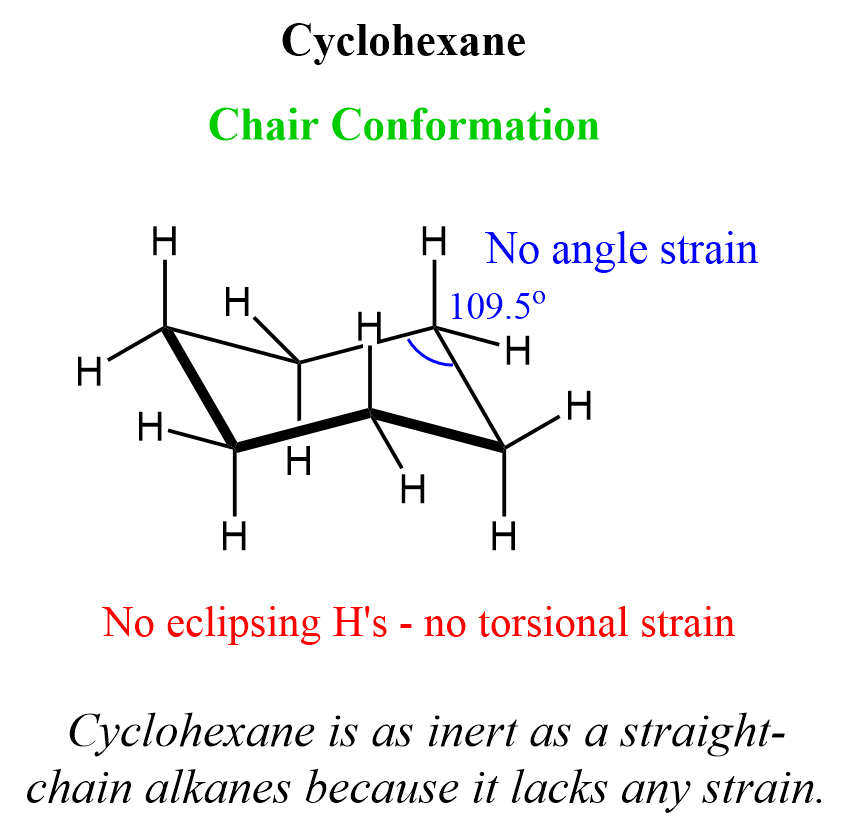
What are the bond angles in the chair conformation of cyclohexane?
Answer: 109.5°
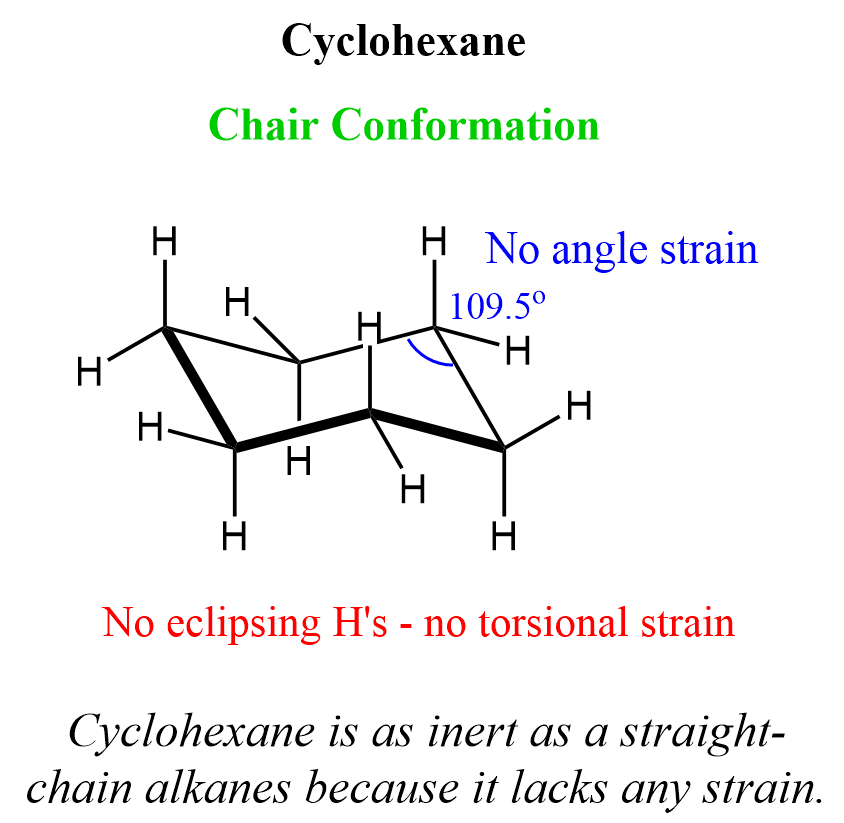
Why does the chair conformation of cyclohexane have zero ring strain?
Answer: All bond angles are 109.5° and there are no eclipsing interactions between C-H bonds.
What causes torsional strain in the boat conformation of cyclohexane?
Answer: Eclipsing interactions between C-H bonds and the close proximity of "flagpole" hydrogens.

What is the "twist boat" conformation and why does it form?
Answer: A slightly skewed boat conformation that forms to reduce unfavorable interactions in the regular boat.

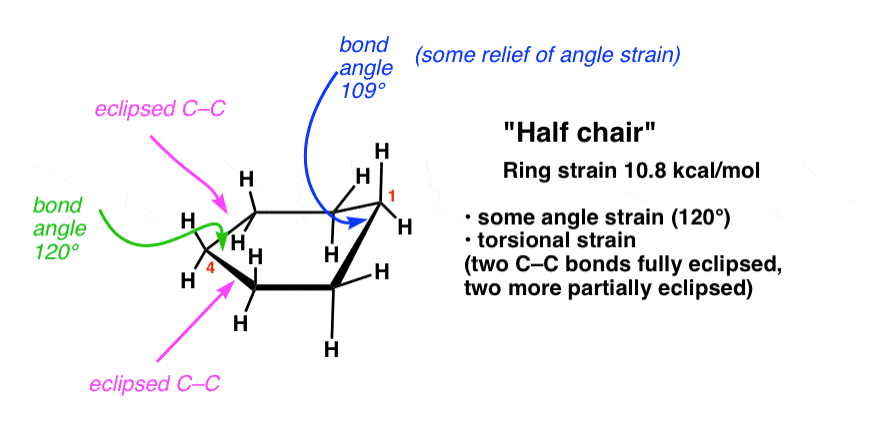
What is the highest energy conformation in the cyclohexane conformational interconversion?
Answer: The half-chair conformation.
What are the two types of C-H bonds in the chair conformation of cyclohexane?
Answer: Axial bonds and equatorial bonds.
How many axial and how many equatorial bonds are there in a chair conformation of cyclohexane?
Answer: Six axial bonds and six equatorial bonds.
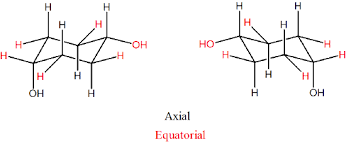
Describe the arrangement pattern of axial and equatorial bonds in cyclohexane.
Answer: Each carbon atom has one axial and one equatorial hydrogen, and each face of the ring has three axial and three equatorial hydrogens in an alternating arrangement.
What is a "ring flip" in cyclohexane?
Answer: The interconversion between two chair conformations.
What happens to axial and equatorial positions during a ring flip?
Answer: Axial positions become equatorial and equatorial positions become axial.
True or False: During a ring flip, all axial positions become equatorial and all equatorial positions become axial.
Answer: True
What is the energy barrier for the ring flip in cyclohexane?
Answer: It is fairly small, allowing chair conformations to readily interconvert at room temperature.
Are the two chair conformations of monosubstituted cyclohexane equally stable?
Answer: No, they are not equally stable.
Which position (axial or equatorial) is generally more stable for substituents on cyclohexane?
Answer: The equatorial position is generally more stable.
What is a 1,3-diaxial interaction?
Answer: A repulsive interaction between axial substituents on C1 and C3 (or C1 and C5) that are close in space.
How does the size of a substituent affect the energy difference between axial and equatorial conformations?
Answer: Larger substituents generally give rise to a larger energy difference between axial and equatorial conformations.
What type of interaction occurs when a methyl group is in the axial position of cyclohexane?
Answer: Gauche (or 1,3-diaxial) interactions.
How many gauche interactions does axial methylcyclohexane have?
Answer: Two gauche interactions.
What is the energy cost of each gauche interaction in methylcyclohexane?
Answer: 0.9 kcal/mol (3.8 kJ/mol).
True or False: A tert-butyl group prefers the axial position in cyclohexane.
Answer: False, it strongly prefers the equatorial position.
Which substituent has the largest axial-equatorial energy difference according to the table?
Answer: tert-butyl (-C(CH₃)₃) with 5.4 kcal/mol.
In cis-1,3-dimethylcyclohexane, which arrangement of methyl groups is more stable?
Answer: The diequatorial arrangement (both methyls in equatorial positions).
Why is there great steric interference when there are two large substituents in axial positions on C1 and C3?
Answer: Because they are close in space and their electron clouds repel one another (1,3-diaxial interaction).
Compare the stability of the two conformations of trans-1,3-dimethylcyclohexane.
Answer: Both conformations are equivalent in stability since they both contain one methyl in axial and one in equatorial position.
Which is more stable, cis-1,3-dimethylcyclohexane or trans-1,3-dimethylcyclohexane, and why?
Answer: Cis-1,3-dimethylcyclohexane is more stable by 1.8 kcal because it can adopt a conformation with both methyl groups in equatorial positions.
What is the energy difference between the cis and trans isomers of 1,3-dimethylcyclohexane?
Answer: 1.8 kcal (the energy difference between axial and equatorial methyl groups).
True or False: All disubstituted cyclohexanes exhibit geometric isomerism.
Answer: False. To show cis-trans isomerism, at least 2 ring carbon atoms must have 2 different groups attached.
What is required for a cycloalkane to show cis-trans isomerism?
Answer: At least two ring carbon atoms must have two different groups attached.
When two substituents of different sizes cannot both be equatorial, which rule determines their positions?
Answer: The larger group goes equatorial, and the smaller goes axial.
Why do extremely bulky groups like tert-butyl always prefer the equatorial position?
Answer: Due to severe 1,3-diaxial interactions when in the axial position.
What happens when two tert-butyl groups cannot both take equatorial positions in cyclohexane?
Answer: The molecule is forced into the twist boat conformation, which will be less crowded and lower in energy.
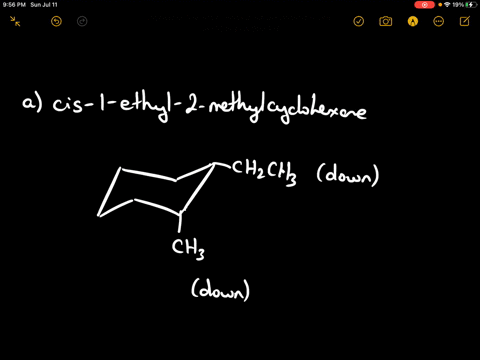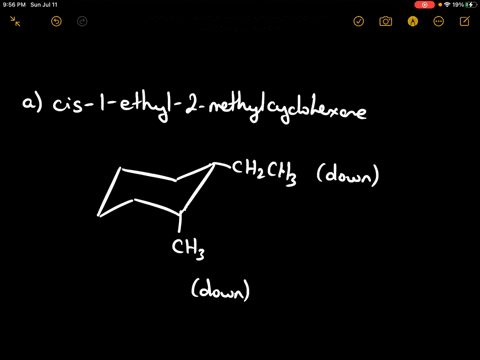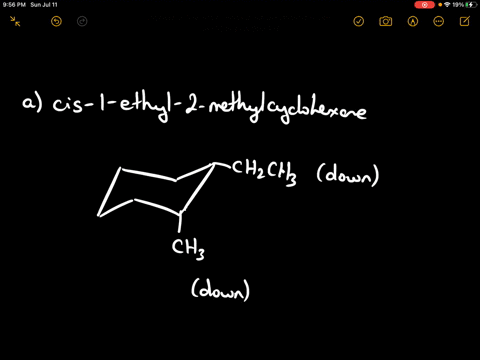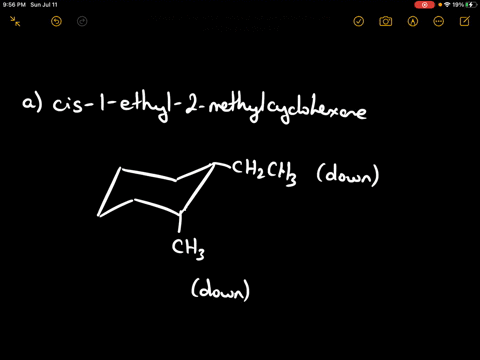
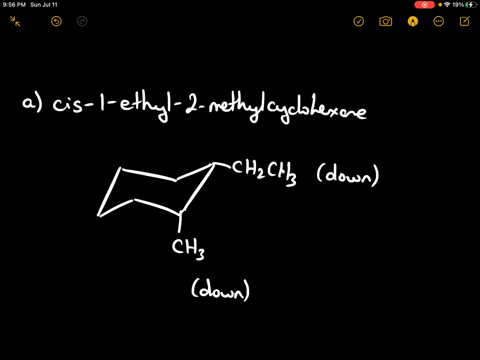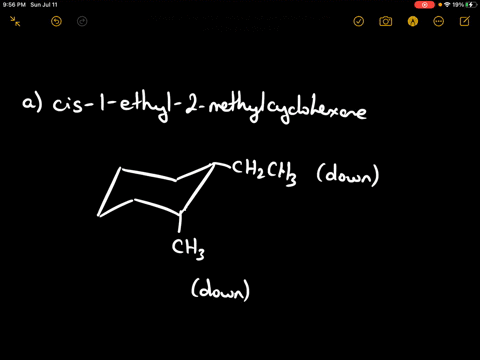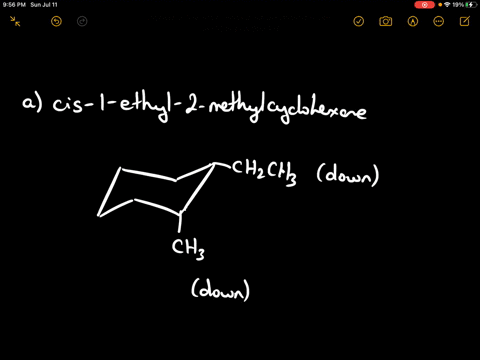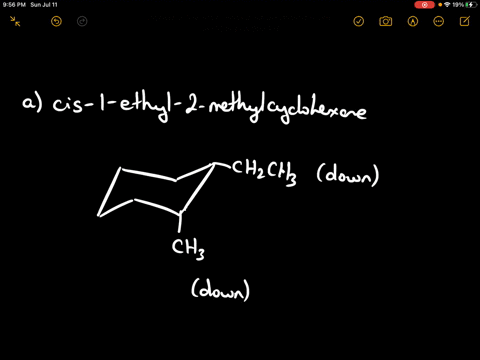
Draw the more stable chair conformation of cis-1-ethyl-2-methylcyclohexane and explain your reasoning.
Answer: The more stable conformation will have both substituents in equatorial positions. Since they are cis and on adjacent carbons, they must be on the same side of the ring. Ethyl is larger than methyl, but both can adopt equatorial positions in this case.
How many different chair conformations can cyclohexane adopt?
Answer: Two chair conformations that interconvert through ring flips.
Why does a fluorine substituent have a smaller axial-equatorial energy difference than a methyl group?
Answer: Because fluorine is smaller and creates less steric hindrance in the axial position.
What is the relationship between the structure of a cycloalkane and its reactivity?
Answer: More strained cycloalkanes (like cyclopropane) are more reactive due to their higher energy content.
True or False: The equatorial position is always more stable than the axial position for any substituent.
Answer: True for most substituents, though some very small substituents may have minimal energy differences.
Why does trans-1,4-di-tert-butylcyclohexane adopt a twist boat conformation?
Answer: Because in a chair conformation, one tert-butyl group would have to be axial, creating severe 1,3-diaxial interactions.