(3.2.1) IC - Periodicity
1/13
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
14 Terms
Where are metals and non-metals split on the periodic table?
1. They are split along the staircase line, with metals on the left, and non-metals on the right.
2. Elements that touch this line have a combination on metallic and non-metallic properties, known as metalloids.
e.g. silicon is non-metal, but it looks shiny and conducts electricity, although not as well as a metal.
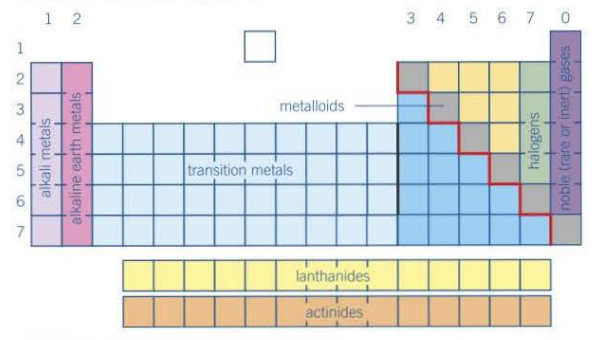
What does the group number tell about the elements in that group?
1. They all have the same number of electrons on their outer shell.
2. The exception to this rule is group 0, which have 8 electrons in their outer shell, a full outer shell, apart from helium, which has 2.
3. All elements in a group have similar properties.
What does the period number tell about the elements in that group?
They all have the same number of electron shells, ignoring s and p-sub shells.
How are s, p, d and f-blocks split amongst the periodic table?
1. All the elements that have their highest energy electrons in s-orbitals are in the s-block.
2. All the elements that have their highest energy electrons in p-orbitals are in the p-block
3. All the elements that have their highest energy electrons in d-orbitals are in the d-block
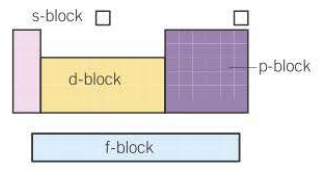
What are the reactivity trends like comparing metals (s-block) to non-metals?
1. In the s-block, as you go down a group the elements get more reactive.
2. To the right of this, as you go down, the non-metal elements get less reactive.
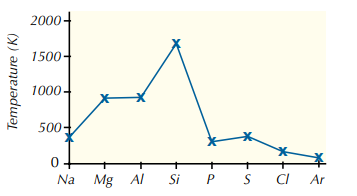
What is the trend for the melting and boiling point across a period? (use the example of period 3)
1. Sodium, magnesium and aluminium are metals, so have metallic bonding.
2. Their melting points increase across the period as the metallic bonding gets stronger as the metal ions have an increasing nuclear charge (positive), an increasing number of delocalised electrons and a decreasing radius.
3. Silicon is macromolecular, with a tetrahedral structure and strong covalent bonds linking all the atoms together, and a lot of energy is needed to break those bonds, so silicon has a high boiling point.
4. There is then a large drop with phosphorus (P4), silicon (S8) and chlorine (Cl2).
5. P4, S8 and Cl2 are all molecular substances, so their melting points depend upon the strength of van der Waals forces between the molecules.
6. van der Waals forces are weak and easily overcome so the elements have low boiling points.
7. Sulphur is the biggest molecule, so it’s got a higher melting point than phosphorus or chlorine.
8. Argon has a very low melting point because it exists as individual atoms, resulting in very weak van der Waals forces.
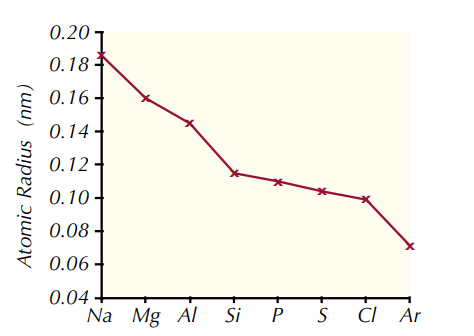
Why do atomic radii decrease across a period?
1. As the number of protons increases, the positive charge of the nucleus increases.
2. This increased charge pulls the electrons in closer to the nucleus, making the atomic radius smaller.
3. There are no additional electron shells to increase shielding, as the extra electrons that are gained across a period are added to the outer energy level.
Why do atomic radii increases down a group?
1. With every increment down a group, an electron shell is added.
2. This increases the distance between the outer electrons and the nucleus, reducing the power of attraction.
3. More shells also increase the electron shielding.
4. This reduces nuclear attraction, and atomic radii increase.
What is the first ionisation energy?
The energy needed to remove 1 electron from each atom in 1 mole of gaseous atoms to form 1 mole of gaseous 1+ ions.
What is the equation for the first ionisation energy, using X as the example element?
X(g) → X+(g) + e-(g)
What is the trend for the first IE across a period and why?
1. It increases.
2. This is because there is a greater nuclear charge as there are more protons.
3. The outer electron is on the same shell, so there is the same amount of shielding.
4. This means that there is a stronger attraction from the nucleus for outer electrons.
5. So more energy is required to overcome this attraction and remove an electron.
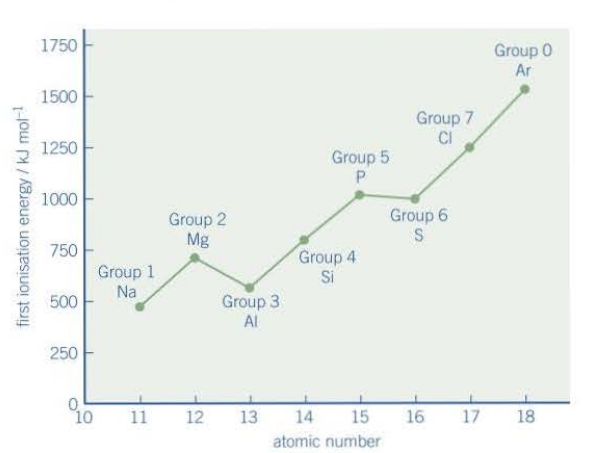
What is the trend for first IE down a group?
Itp decreases as the nuclear attraction between the nucleus and outer electron reduces and increasing amounts of shielding means less energy is required to remove the outer electron.
Why is there a drop between groups 2 and 3 on a graph showing the increasing IE across a period?
1. The group 3 element’s outer electron is in the p sub-shell, whereas the group 2 element’s outer electron is in the s sub shell.
2. The p sub-shell is higher in energy and has more shielding from the s sub shell.
3. This means there is a weaker nuclear attraction, so less energy is required to remove it.
Why is there a drop between groups 5 and 6 on a graph showing the increasing IE across a period?
1. An electron in a pair will be easier to remove than one in an orbital in its own as it is already being repelled by the other electron, so less energy is needed to remove it.
2. The element in group 5 has no paired electrons in its p sub shell, as each p electron is in a different orbital.
3. The element in group 6 has two of its p electrons shared, so they’ll repel each other, so less energy is required to remove it.