4.4.1.3 The development of the model of the atom
0.0(0)
Card Sorting
1/16
Last updated 9:06 AM on 12/14/22
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
17 Terms
1
New cards
A scientific theory is a
well-substantiated explanation of some aspect of the natural world, based on a body of facts that have been repeatedly confirmed through observation and experiment
2
New cards
1800 - Dalton
Dalton said everything was made of tiny spheres (atoms) that could not be divided
All matter is made of atoms
Atoms cannot be broken down into anything simpler
All the atoms of a particular element are identical to each other and different from the atoms of other elements
All matter is made of atoms
Atoms cannot be broken down into anything simpler
All the atoms of a particular element are identical to each other and different from the atoms of other elements
3
New cards
1897 - JJ Thomson
**discovery of electrons**
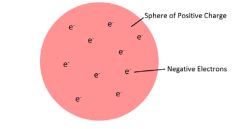
JJ Thomson discovered the electron The Plum Pudding Model was formed
the positively charged matter evenly spread about (the pudding) and electrons buried inside (the plums).
The discovery of the electron led to the plum pudding model of the atom
. The plum pudding model suggested that the atom is a ball of positive charge with negative electrons embedded in it.
The overall charge of an atom is neutral, so the negative electrons were dispersed through the positive “pudding” to cancel out the charges.
JJ Thomson discovered the electron The Plum Pudding Model was formed
the positively charged matter evenly spread about (the pudding) and electrons buried inside (the plums).
The discovery of the electron led to the plum pudding model of the atom
. The plum pudding model suggested that the atom is a ball of positive charge with negative electrons embedded in it.
The overall charge of an atom is neutral, so the negative electrons were dispersed through the positive “pudding” to cancel out the charges.
4
New cards
1909/11 - Rutherford
- Rutherford realised most of the atom was empty space
5
New cards
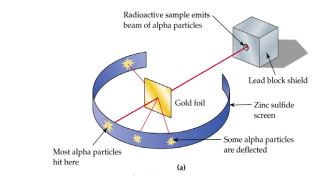
Gold Foil Experiment
scientist took a piece of Gold foil- this is because gold can be hammered out into very thin sheets only a few atoms thick
they fired tiny alpha particles at the gold foil
==Most 𝛼 particles went straight through, So most of atom is empty space==
==Some 𝛼 particles were slightly deflected So nucleus/ center of the atom must be positively charged, and that repelled positive 𝛼==
==Few 𝛼 particles bounced back so most mass was concentrated at the Centre of the atom==
\
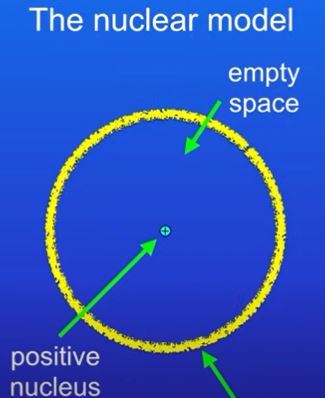
Now there is a positive nucleus at the centre of the atom, and negative electrons existing in a cloud around the nucleus .
Their results replaced the plum pudding model to show a small positively charged nucleus surrounded by mostly empty space with electrons dispersed through it
\
used these results to construct **nuclear model**
they fired tiny alpha particles at the gold foil
==Most 𝛼 particles went straight through, So most of atom is empty space==
==Some 𝛼 particles were slightly deflected So nucleus/ center of the atom must be positively charged, and that repelled positive 𝛼==
==Few 𝛼 particles bounced back so most mass was concentrated at the Centre of the atom==
\
Now there is a positive nucleus at the centre of the atom, and negative electrons existing in a cloud around the nucleus .
Their results replaced the plum pudding model to show a small positively charged nucleus surrounded by mostly empty space with electrons dispersed through it
\
used these results to construct **nuclear model**
6
New cards
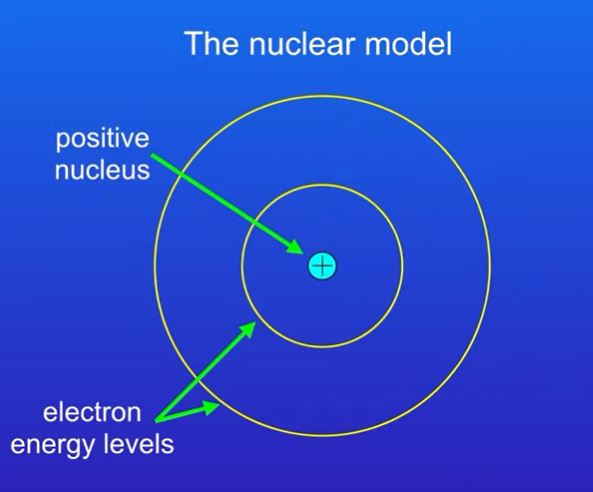
1913 – Bohr
Bohr proposed that the electrons orbit the nucleus at specific distances
accepted because it agreed with results from other scientist
accepted because it agreed with results from other scientist
7
New cards
, James Chadwick
the ‘nucleus’ was an accepted scientific idea, James Chadwick providedevidence to prove neutrons existed (don’t need specifics)
8
New cards
summary
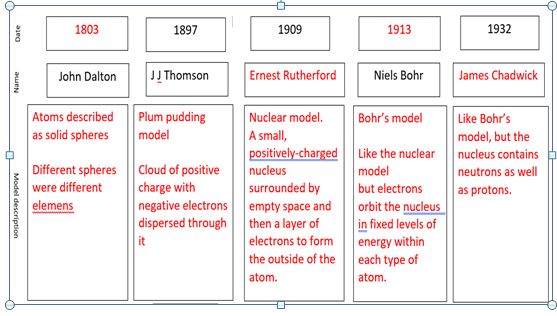
9
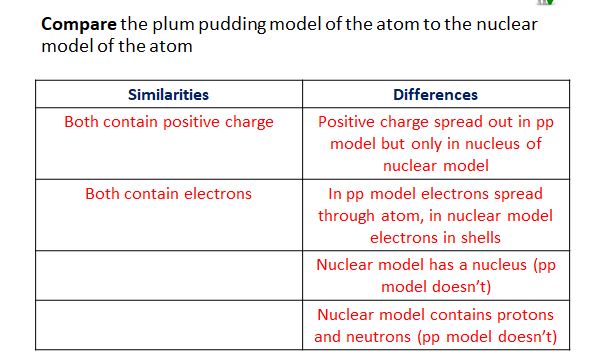
New cards
Compare the plum pudding model of the atom to the nuclear model of the atom
Both models contain a positive charge however in the plum pudding model it is spread throughout the atom whereas in the nuclear model it is only in the nucleus.
Electrons in plum pudding model are spread throughout the atom but in the nuclear model they are in electron shells
The nuclear model shows the atom to have proton, neutrons and electrons where as the plum pudding model only has electrons.
Nuclear model has a nucleus but the plum pudding model does not.
Electrons in plum pudding model are spread throughout the atom but in the nuclear model they are in electron shells
The nuclear model shows the atom to have proton, neutrons and electrons where as the plum pudding model only has electrons.
Nuclear model has a nucleus but the plum pudding model does not.
10
New cards
what would you expect from the plum pudding model
Based on ‘the plum pudding model’ atom we would expect that the alpha particles would pass straight through the atom as charge is evenly spread out through the atom and also overall neutral.
11
New cards
gold foil experiment
Rutherford used a radioactive source to fire positively
charged, alpha particles at a thin gold foil.
MOST alpha particles went straight through. this means The atom is mostly empty space.
SOME were deflected (bent off course) this means The alpha particles were passing by something positive – the fact that only a few were deflected suggested the positive charge was very small
A very small number came straight back this means All the positive charge was concentrated in a small space at the centre of the atom
Rutherford concluded that the gold atoms are mostly empty space with a positively charged nucleus that contains nearly all the mass of the atom.
charged, alpha particles at a thin gold foil.
MOST alpha particles went straight through. this means The atom is mostly empty space.
SOME were deflected (bent off course) this means The alpha particles were passing by something positive – the fact that only a few were deflected suggested the positive charge was very small
A very small number came straight back this means All the positive charge was concentrated in a small space at the centre of the atom
Rutherford concluded that the gold atoms are mostly empty space with a positively charged nucleus that contains nearly all the mass of the atom.
12
New cards
Why did the findings change the atomic model?
Scientists quickly accepted Rutherford’s proposal because:
Rutherford was a very well-respected scientist
He had lots of evidence/data because he took thousands of readings. What does this suggest?
Rutherford was a very well-respected scientist
He had lots of evidence/data because he took thousands of readings. What does this suggest?
13
New cards
Development of the model of the atom
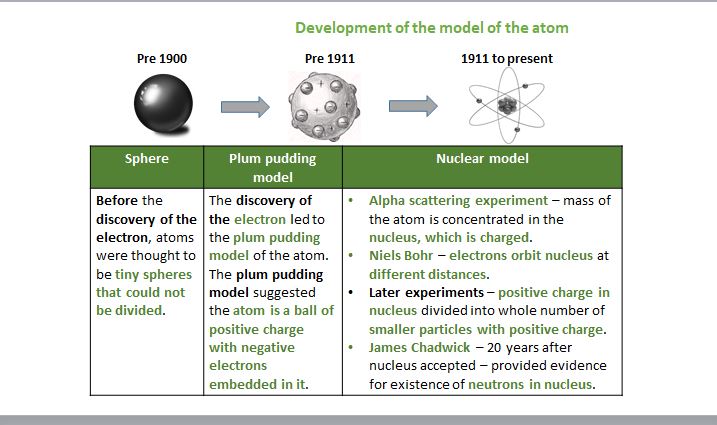
14
New cards
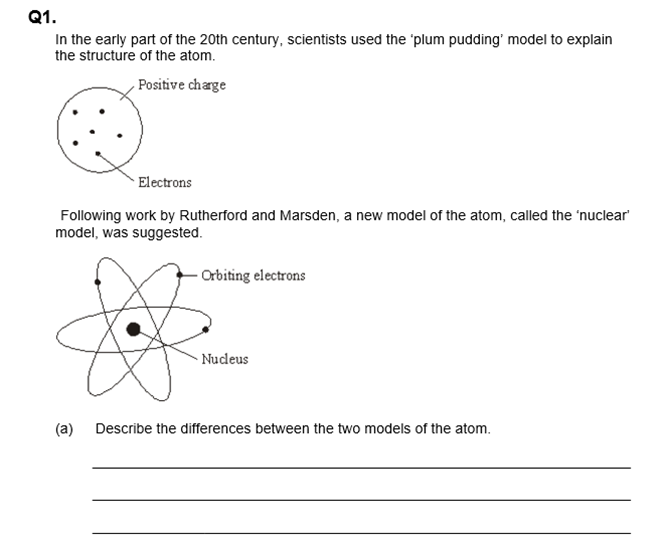

15
New cards


16
New cards


17
New cards