Chemistry - Unit 8 - Covalent Bonding
1/59
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
60 Terms
hydrides
compounds with hydrogen
general rule for boiling point
it increases as we move down the rows of periodic table
what happens if boiling point increases
greater IMF strength
what happens if we move down the periodic table
more principal energy levels thus greater atomic radii
molecules with greater surface area have greater London forces
What is required for Hydrogen Bonding to occur
molecules contains extreme polar bonds between Hydrogen and nitrogen or oxygen or fluorine
there needs to be a covalent bonds in the molecule H-F, H-N, or H-O
once of the molecules bonding needs an H-NOF bond so there can be Hydrogen bonding between CO molecule and H-N molecule
hydrogen bonding
a third IMF which is much stronger than London and Dipole-Dipole forces
it is an attraction or force not a type of bond
can occur within a molecule or between
Has to be between H-NOF bonds
it can bond with another molecule, not with H-NOF bond, but has to attract with NOF lone pair from other molecule (non H-NOF)
How are hydrogen bonding drawn
as a dotted line between molecules, never inside the molecule
the hydrogen bonding is always from hydrogen to a lone pair of N/O/F of other molecule
water as hydrogen bonding
water can from max of 4 hydrogen bonds
they form some hydrogen bonds but not all four, when heating up water the hydrogen bonds break and stay as clumps of water molecules —> why ice floats in liquid water
ice hydrogen bonding
ice has an arrangement with 4 hydrogen bondings forming a tetrahedral structure
this is why ice is less dense than water as in every ice every water molecule is spread out from neighbouring molecules with the distance of a full hydrogen bond
vapor pressure
increasing volatility increases the vapor pressure
also increases with the temperature
Polarity and IMF strength
polarity influences IMF strength
only if the size is controlled, more polar means more IMF
if molecules are small
dipole-dipole forces
between two poles, when we have polar molecules one end is a little more polar while the other is less
electrostatic attraction between opposing charges end of polar molecules
London dispersion
electrostatic attraction between instantaneous dipoles - always present
water solubility
the ability to dissolve in water
size and polarity influence if it is soluble
non-polar molecules are not soluble
C6H13NH2 is polar except the size is too big, thus only a small part of the molecule can form hydrogen bonds - thus a -not soluble, big part of its IMF are non-polar London Forces.
anything past 4 carbon is unable to mix with water for organic compounds
long carbon chains causes less water solubility
it has to form attractions with water, from hydrogen bonds with water
eg, CH3CHO does not have hydrogen bonds but can form hydrogen bonds with water as water has lone pairs
this is most relevant for O and N
IMF vs Intramolecula rbonds
covalent, Ionic, and Metallic bonds are stronger than London, Dipole-Dipole Forces, and Hydrogen bonding
hydrogen accepting and donating
this term refers to two molecules of hydrogen bonding, one with and one without H-NOF bonds
ion-dipole reaction
when a molecule is polar it is attracted to the dipole of water creating an ion-dipole reaction
less polar solvents
some solvents are polar but not enough to dissolve in water, they are polar in other solutes such as benzene, ether
other forms of graphite
Graphene and
a single layer of graphite
C60 Buckmister Fullerene "or Bucky Ball
Graphene
each carbon atom is covalently bonded to 3 other carbon atoms, angle between carbon atoms is trigonal planer 120
electrons delocalised across layers
thus conductivity very high,
highest known thermal conductivity,
very strong and flexible with a high melting point
can be used for voltaic cells and computers, which is very useful
The Bucky Ball
another form of Carbon, found in outer space or hit with lasers in the lab
carbon atoms are bonded together in series of pentagons and hexagons, carbon atoms are always bonded to three other atoms, 6 or 5 rings
a distorted trigonal planar,
electrons delocalized around the ball )individual molecule) but electrons can not pass between another molecule next to it
poor thermal and electrical conductivity
it is light and strong - non-polar, thus soluble in non-polar solvents
low melting point - sublimes
also works as a lubricant as the layers can”roll”past each other
can act as a cage for smaller atoms
SIlicon
structure is identical to carbon or diamond, except that the atoms are bigger. giant covalent structure
has 4 silicon atoms bonded
very hard with high melting point, as it has a strong covalent bond in al directions
it is a semi-conductor s it is a metalloid,
important to computer and solar energy industry
Silicon Dioxide
a Giant Molecular structure, all covalent bonds
tetrahedral shape every si atom has 4 oxygen atoms and every oxygen atom is bonded to 2 si atoms
the ratio for silicon to oxygen is 1Si: 2O
extremely hard with high melting points
can be used in electronics, like watches, unique electrical conductivity properties
insoluble in water
Graphite
each carbon atoms are covalently bonded to 3 other carbon atoms - it is found in layers, one carbon atom has 1 double bond and 2 single bonds meaning multiple positions = resonance more like 1,5 bonds with delocalised electrons all in the rings and in between each layer
they have London forces in between the layers
trigonal planer
poor thermal conductivity, because of the space between layers
high electrical conductivity because of delocalized electrons
very soft and brittle, acts as a lubricant
it works on paper, because layers slide past each other, due to weak London forces between layers
diamond
each carbon atom is covalently bonded to 4 other carbon atoms
tetrahedral 109.5
localized electrons, no mobile electrons
no electrical conductivity
extremely high thermal conductivity
very strong because no IMF’s, high melting point with strong bonds
polar and non-polar covalent bonding
non-polar have a small electronegativity between two bonding elements, thus electron pairs are shared more or less evenly
large electronegativity has polar pairs, where electron pairs are shared unevenly between two atoms
metallic bond
electrostatic attraction between a lattice of positive metal cations and delocalized valence electrons moving between them
used for metals and alloys
covalent bond
electrostatic attraction between 2 positive nuclei and the shared electron pairs between them
used for nonmetal elements, all molecules, all organic compounds, giant covalent
covalent bond strength measured via bond enthalpies, the energy required to break 1 mole of a bond in gas phase
ionic bond
electrostatic attraction between oppositely charged ions
used for ionic compounds, salts and minerals
what is the octet rule
the atoms like to have eight electrons only in their full outer shells.
exceptions for the octet rule
beryllium and boron have 2 and 3 valence electrons and, thus, are unable to form molecules with 2 and 3 covalent bonds, leaving them with incomplete octets.
for them to form a complete octet, other species have to donate electron pairs through coordinate covalent bonding
coordinate covalent bonding
a bond between two atoms in which the electron pairs is donated from one atom, H+ and O donates two electrons to form a bond
it can be spotted if one element usually forms 2 bonds but forms three, thus they donated an electron
if it makes more than normal pairs, it is donating electrons, coordinate covalent bond
VSEPR theory
in the valence shell, electron pairs will repel each other as far as possible, due to electron repulsion
electron domain geometries
on electron domain:
single bond
double bond
triple bond
nonbonding electron pair
if there are non-bonding electron pairs, they repel more than bonding pair of electrons since they do not have an atom pulling it on either side.
bond angles and shapes SL
2 domains:
linear 180
3 domains:
no lone pairs, trigonal planar 120
1 lone pair; bent 117, remove three for each lone pair
4 domains:
no lone pairs, tetrahedral 109.5
1 lone pair, trigonal pyramidal 106.5
2 lone pairs, bent 103.5
how to find the polarity of the molecule
are the bonds non-polar, the EN difference less than 0.4
what is the molecular geometry, drawn in 3D
are the bonds arranged so that they oppose each other? Do they look like they cancel out, or arranges asymmetrically - thus what is the net dipole
rule for polarity in organic molecules
C-H = non-polar
C-N/C=N = slightly polar
C-O/C=O, C-Cl, C-F = polar
N-H = very polar
O-H = very polar
Organic molecules
My - 1 carbon
Elephant - 2 carbon
Poops - 3 carbon
Bananas - 4 carbon
Pentane - 5 carbon
Hexane - 6 carbon
Alkane - CnH2n+2
Alkene - CnH2n
Alkynes - CnH2n-2
Alcohol - CnH2n+1OH
In organic molecules, if the chain is long the molecule is not polar
what are the 7 steps for complex lewis structures
how many valence electrons do we have to work with, O2 would be 12
to satisfy the octet rule each need 8, so how many electrons do we need in total
subtract what we need by what we have to see how many electrons are shared
divide this number for shared electrons by two to see how many bonds we have in total
draw bonds
draw lone pairs to give octets
check if all available electrons are used
if the number of bonds does not make sense, then an extended octet
polytomic ions
they are held together by covalent bonds, the whole ion makes ionic bonds with other molecules
NH4+ = ammonium
NO3- = Nitrate
OH- = Hydroxide
HCO3- = hydrogen carbonate
SO4 2- = Sulfate
CO3 2- = Carbonate
PO43- = Phospate
resonance
resonance occurs when there is more than one possible location for a pi bond, shows that a double bond can be in multiple places
pi bonds are able to delocalise over multiple bonds when there are multiple parallel p-orbitals in a row. this creates a more stable, resonance hybrid structure where the charge is distributed evenly over the structure, decreasing electron repulsion forces
this creates 1.5 bonds
bond order
a way of describing the actual, net number. of bonds between to atoms. this includes the partial bonds that exist as a result of electron delocalisation - how strong the bond is
single bond = bond order 1
double bond = bond order 2
Bond Order for molecule =
(# of bonds between resonance atoms) / (# of resonance bond locations)
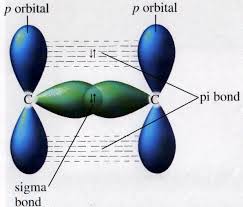
sigma and pi bond
they form when two atomic orbitals overlap head -n. This results in symmetry around the bond axis and electron density between the two bonding nuclei, where electrons spend most time
pi bonds = always p orbitals, form from the sideways overlap of parallel p orbitals and results in electron density above and below the bond axis for each pi bond. , double or triple bonds
sigma bonds = all covalent bonds are sigma bonds, they are the first bond seen, the additional bonds in double bonds are pi bonds
the head on combination of atomic orbitals where the electron density is concentrated along the bond axis
hybridization
Hybridisation refers to combining of atomic orbitals within an atom to form a new set of hybrid orbitals.
the hybridization of 1 s and 1 p orbital creates 2 equal sp orbitals, which helps to explain linear geometries
the hybridization between 1 s and 2 p orbitals creates 3 equal sp2 orbitals which helps to explain trigonal pyramidal electron domain geometries
number of e domains says the type of hybridization
tetrahedral = 4 orbitals 1s +3p = sp3
trigonal planar = 3 orbitals 1s +2p = sp2
linear = 2 orbitals 1s +1p = sp
melting points
the temperature at which a substance changes from solid to liquid, in order to melt the electrostatic attractions between a substance’s particles must be overcome enough that particles can move past each other
boiling point
the temp at which substance changes from liquid to gas below the surface of a liquid
increasing p = increasing bp
in order for a substance to boil, the attractions between particles must be completley overcome in order to separate into gas phase
volatility
how readily a substance evaporates, changes from liquid to gas at the surface of liquid
in order for particles to evaporate, the attractions between them and the particles beneath them must be completley overcome in order to separate into gas phase
stronger attractions between particles low volatility
vapor pressure
the gas pressure created by a substance’s evaporation at a given temperature, increasing volatility increasing vapor pressure
increasing temp increasing vapor pressure
effect of polarity on physical properties
methods to separate mixtures - 7
evaporating and condensing = separates soluble solids from liquids
magnetism = separates iron and steel from non-magnetic materials
filtering = separates insoluble solids from liquids
sieving = separates different-sized solids
decanting = separating two liquids which have different weights
Solvation - substances with different solubility a non polar substance in water does not dissolve then a non polar solvent above water allows the substance to dissolve in the solvent and then separate two substances
recrystallization - impure solids, separating a mixture of 2 solids, add high temp solvent dissolving both solids, as it cools one solids starts to form crystals - then filter to separate
distillation - evaporating two or more liquids, most volatile liquid evaporates most here it passes through a condenser, where it condenses and can recollect have to do multiple rounds
chromatography
a technique that takes many forms and involves separating components of mixtures mainly for identification purposes
separate components based on different polarities
all involve a stationary phase, where the substance/structure does not move
the mobile phase where the substance/mixture moves along the stationary phase
to find the retention factors: (distance from spot)/ (distance of the solvent front)
ion-dipole interactions
an attractive force that results from the electrostatic attraction between an ion and a neutral molecule that has a dipole.
these forces explain the water solubility of ionic compounds
expanded octets
elements on the 1 and 2 can never have an expanded octet but 3rd and above can, the central atoms has one.
electron energy levels converge at higher energies, beginning in the third energy level the d-orbitals are there. Thus elements from the 3p-block can use empty 3d-blocks to have more than 8 electrons
identify:
notice a central atoms bonded to more than its normal number of atoms
try to follow the 7 steps
expanded octet geometries and bond angles
5 bond pairs:
5 bond pairs, 0 lone pairs = trigonal bipyramidal 90 and 120 and 180
4 bond pairs, 1 lone pairs = seesaw, 117, 97, 183
3 bond pairs, 2 lone pairs = T-shaped 183, 87
2 bond pais, 3 lone pairs = linear 180
6 bond pairs:
6 bond pairs, 0 lone pairs = octahedral 90
5 bond pairs 1 lone pair = square pyramidal 87, 183
4 bond pairs, 2 lone pairs = square-planar 90, 180
formal charges
it calculates whether an atom within a molecule or ion carries no charge, positive or negative charge
to determine:
write the lewis structures
count the number of electrons the atom owns
if it is resonance structure choose the one with least formal charge and then consider electronegativity thus which atom would attract electrons
intramolecular bonding
covalent, ionic, or metallic
van der waals forces
london and dipole-dipole forces
what influences the amount of force
size or surface area and polarity of molecules
how do bonds effect te wavelengths absorbed
ozone and oxygen in atmosphere absorbs ultraviolet light
oxygen had a bond order of 2 and ozone 1.5 thus O2 stronger and has mroe enrgy which absorbs smaller wavelengths
Benzene
ring of six carbon atoms, each with a hydrogen atom attached and alternating carbon double bonds