Intermolecular Forces and Molecular Structure
1/3
Earn XP
Description and Tags
Flashcards about intermolecular forces and molecular structure.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
4 Terms
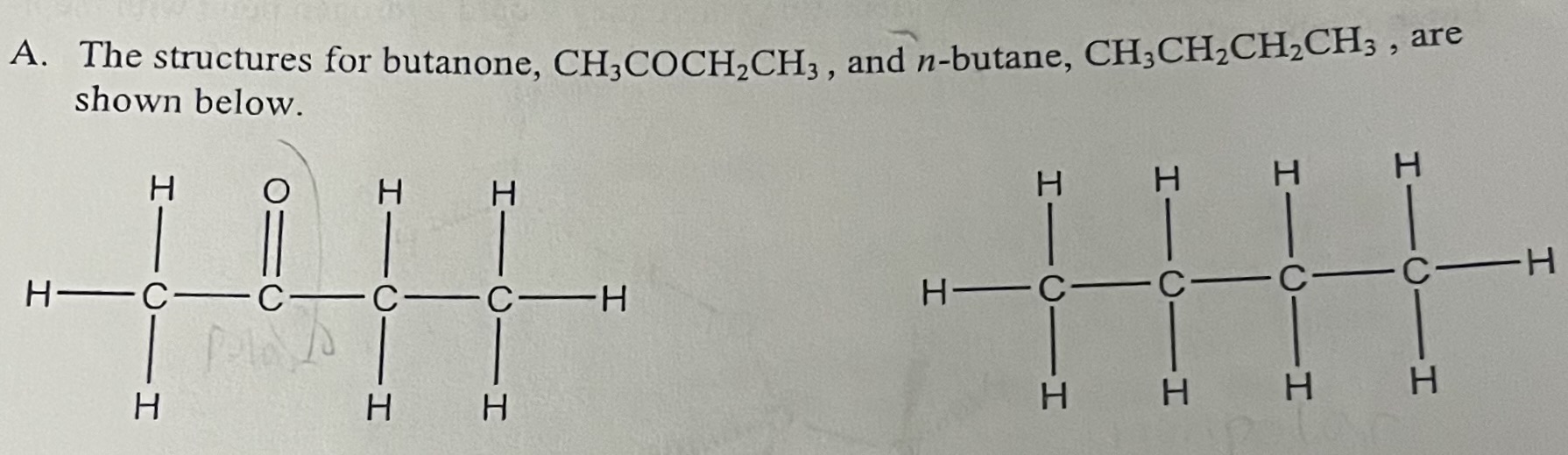
What type of intermolecular forces are present in butanone (CH3COCH2CH3)?
London Dispersion Forces & dipole dipole forces
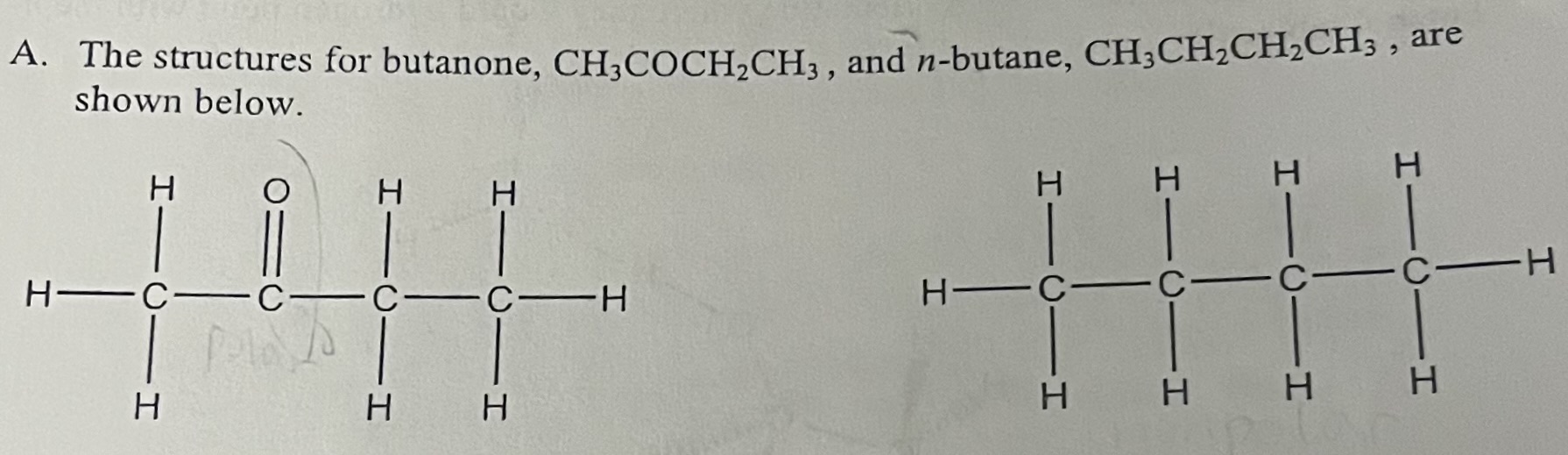
What type of intermolecular forces are present in n-butane (CH3CH2CH2CH3)?
London Dispersion Forces
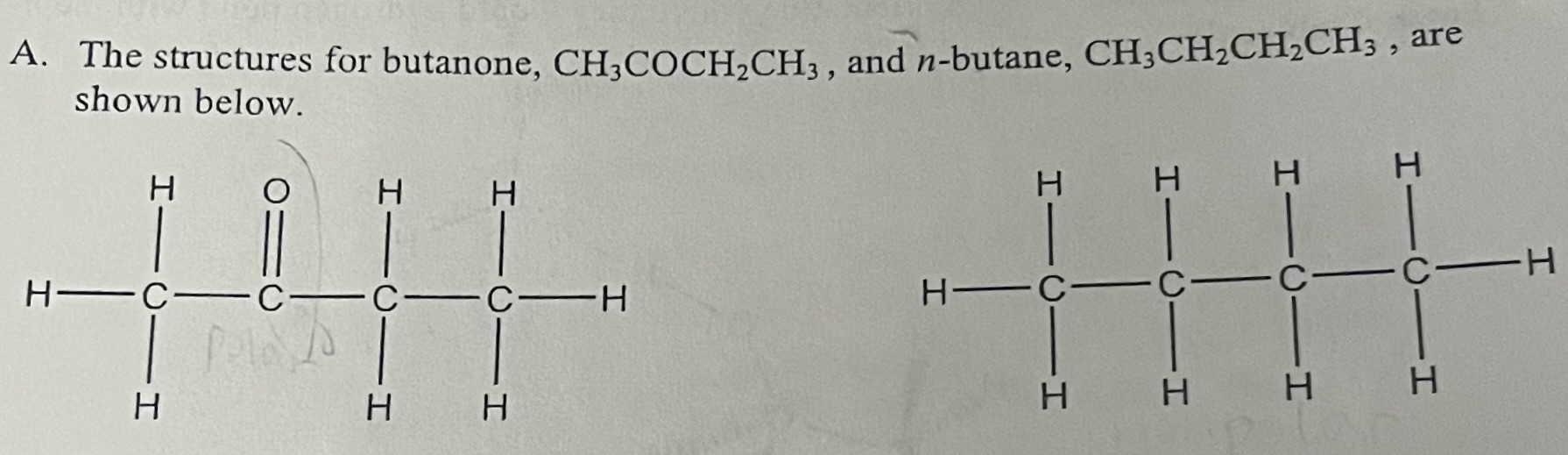
Why is butanone more soluble in H2O than n-butane?
Butanone is more soluble in H2O because it is polar and can hydrogen bond with water due to the lone pair on the oxygen atom.
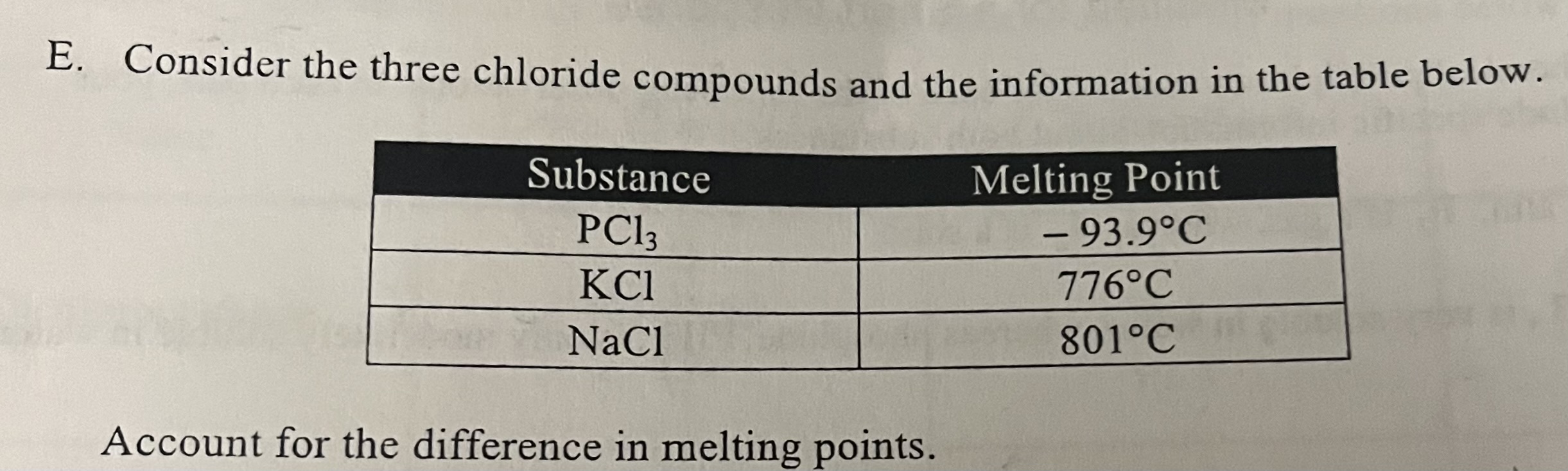
Consider the three chloride compounds and the information in the table below.
PCl3 has a low melting point due to weak intermolecular forces while KCl and NaCl have high melting points due to strong ionic bonds, with NaCl having the highest melting point because of its smaller, more strongly attracted ions.