Bio346 week 2 Photosynthesis and Thylakoids reactions
0.0(0)
0.0(0)
Card Sorting
1/50
Earn XP
Description and Tags
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
51 Terms
1
New cards
2
New cards
How to dissect biology
Recall everything is a process, state, or thing. This is a principle you should use when looking at plants
3
New cards
Project
Go to google scholar or new articles about plants. Ask about later
4
New cards
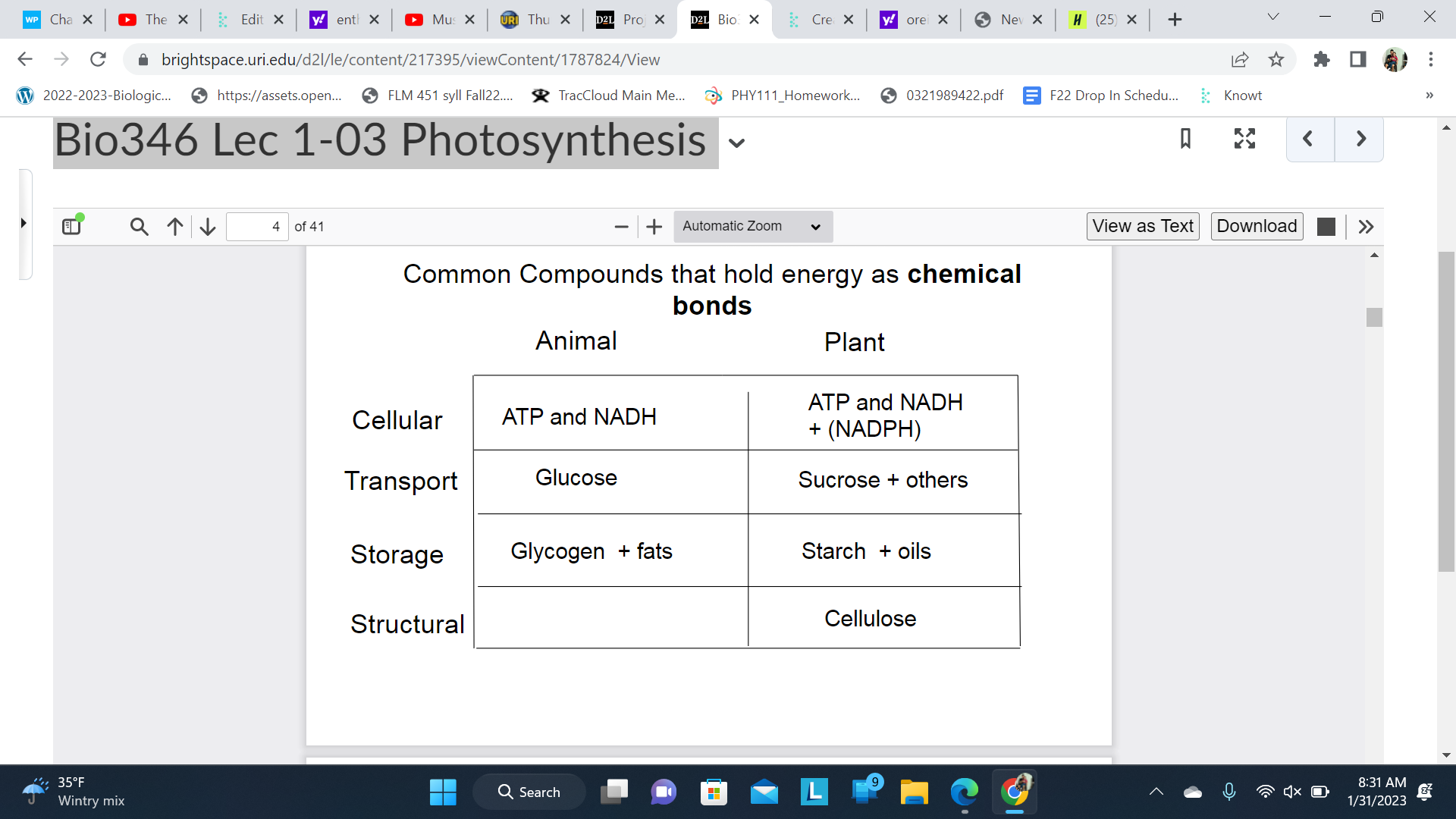
How is energy held in an organism
Chemical Bonds + Electrochemical Gradients
\
Chemical bonds releases most of energy as enthalpy and some entropy ask
\
Electrical Gradients relation to entropy(maintained perhaps)
\
Chemical bonds releases most of energy as enthalpy and some entropy ask
\
Electrical Gradients relation to entropy(maintained perhaps)
5
New cards
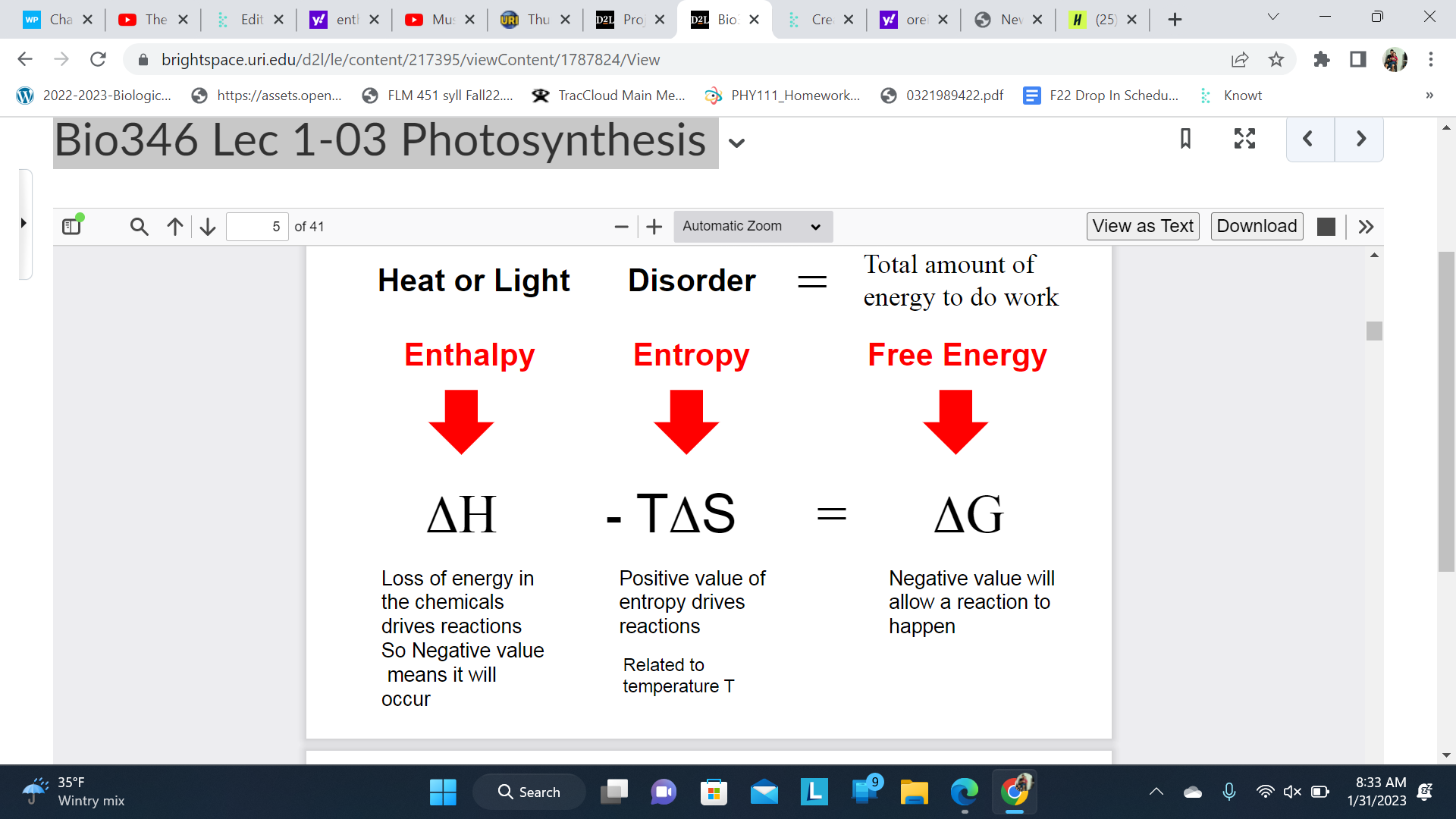
Combine these categories of energy
Enthalpy(Heat and Loss)
\
Entropy(Disorder)
\
Free Energy(Total amount of \n energy to do work)
\
\
Entropy(Disorder)
\
Free Energy(Total amount of \n energy to do work)
\
6
New cards
Free Energy Delta G, represents the amount of work a reaction can do
Predicts whether a reaction will occur
\- Delta G = spontaneous, free energy released to do work (exergonic)
reaction is energetically favorable
\
\+ Delta G = non-spontaneous, free energy consumed
(endergonic)
reaction is energetically unfavorable
\
When you talk about just the release of heat, then a reaction is called exothermic or endothermic
\- Delta G = spontaneous, free energy released to do work (exergonic)
reaction is energetically favorable
\
\+ Delta G = non-spontaneous, free energy consumed
(endergonic)
reaction is energetically unfavorable
\
When you talk about just the release of heat, then a reaction is called exothermic or endothermic
7
New cards
Energy is constantly needed in the cell
Mitosis
Exocytosis
Cell Wall biosynthesis
Ion Uptake/membrane homeostasis
Membrane synthesis
Gene expression
\
\
Organisms require consistent energy input to avoid heading toward equilibrium (=Death!)
Exocytosis
Cell Wall biosynthesis
Ion Uptake/membrane homeostasis
Membrane synthesis
Gene expression
\
\
Organisms require consistent energy input to avoid heading toward equilibrium (=Death!)
8
New cards
Green plants photosynthesize(synthesize sugars or other substances by means of photosynthesis)
The leaf is the primary photosynthetic machine
9
New cards
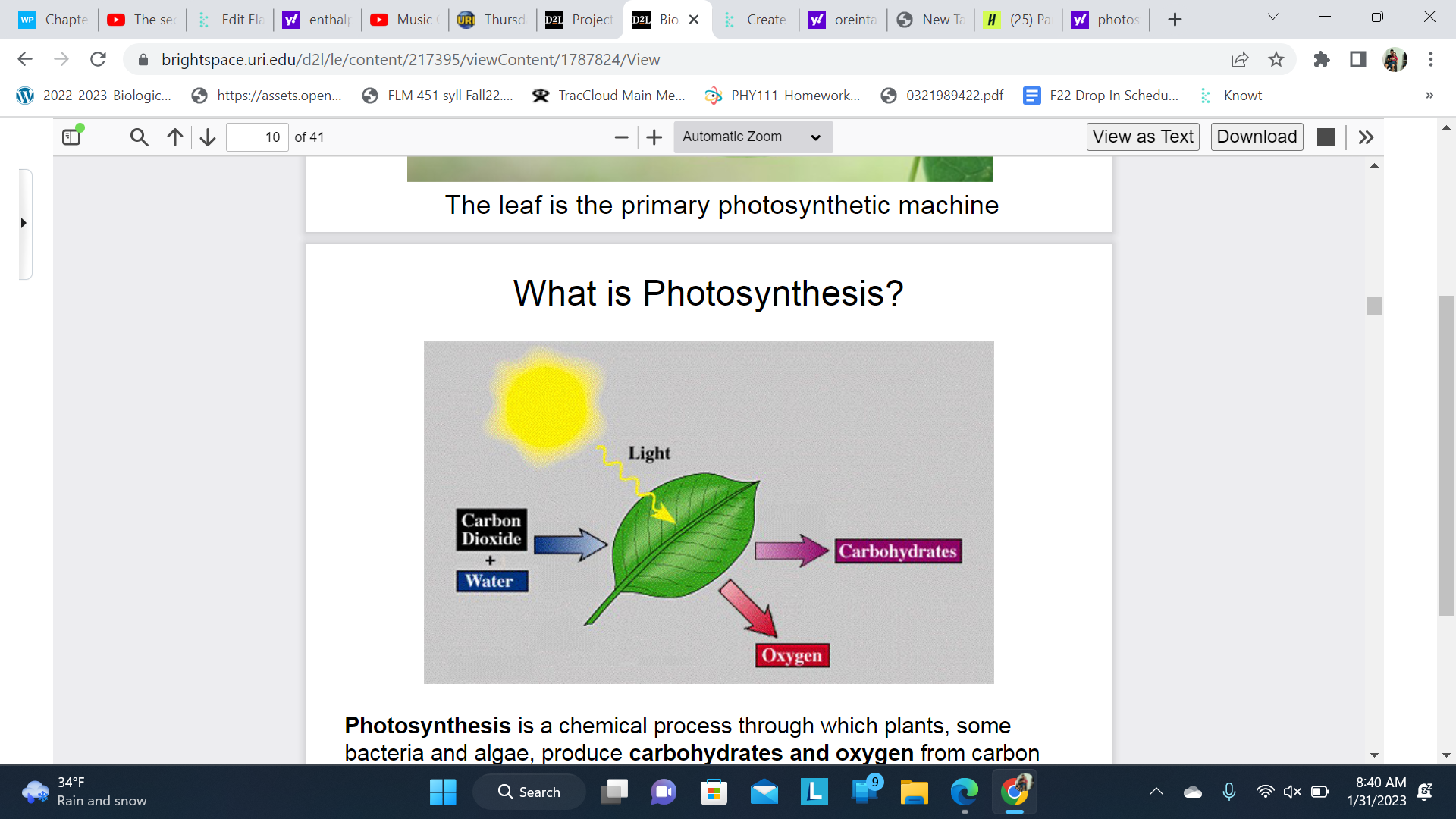
What is photosynthesis
a chemical process through which plants, some bacteria and algae, produce carbohydrates and oxygen from carbon dioxide and water, using light as a source of energy.
10
New cards
There is potential energy in chemical bonds
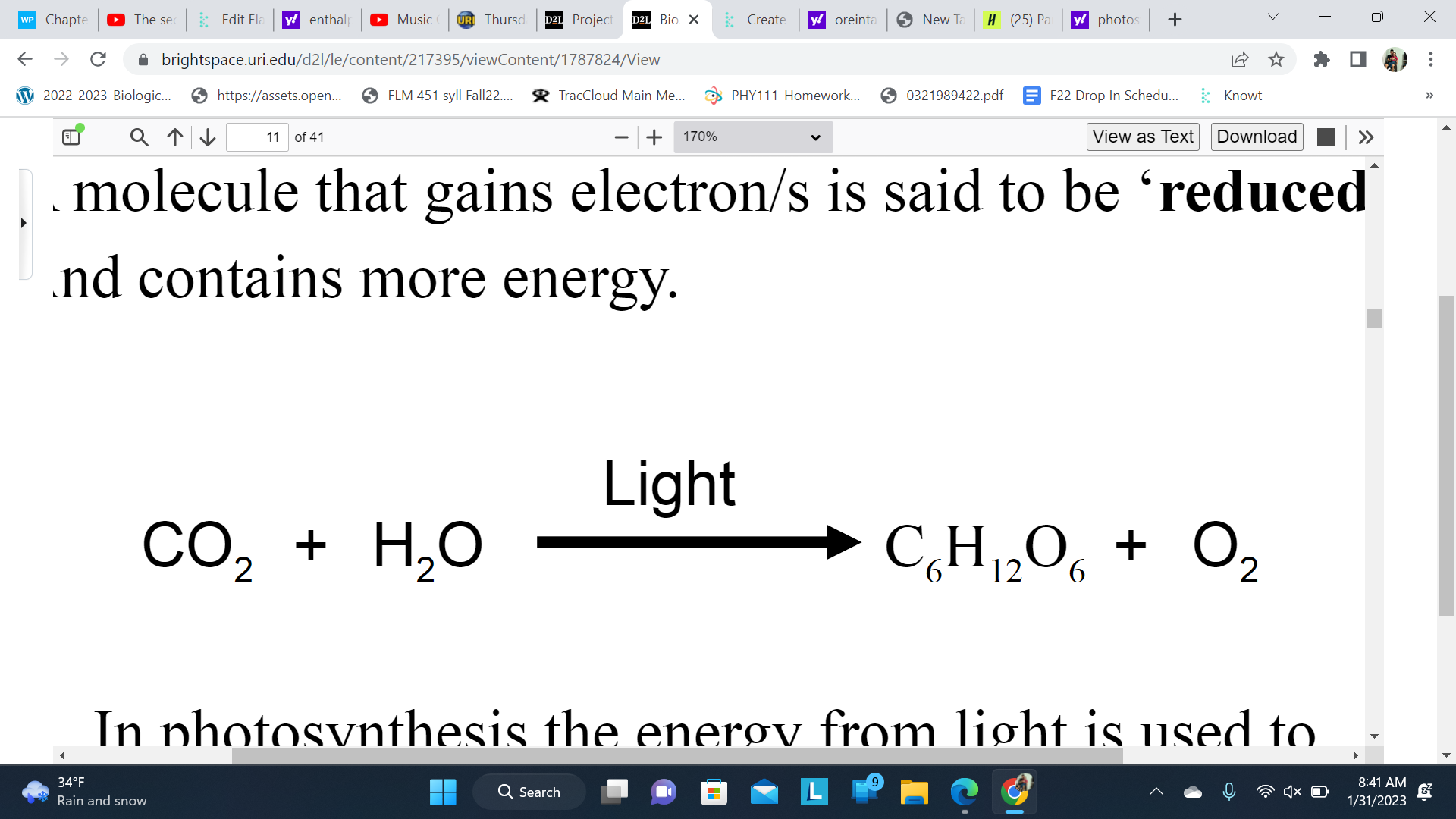
A molecule that gains high energy electron/s is said to be ‘reduced’ And contains more energy
\
In photosynthesis the energy from light is used to ‘reduce’ the Carbon in CO2 to C6H12O
\
In photosynthesis the energy from light is used to ‘reduce’ the Carbon in CO2 to C6H12O
11
New cards
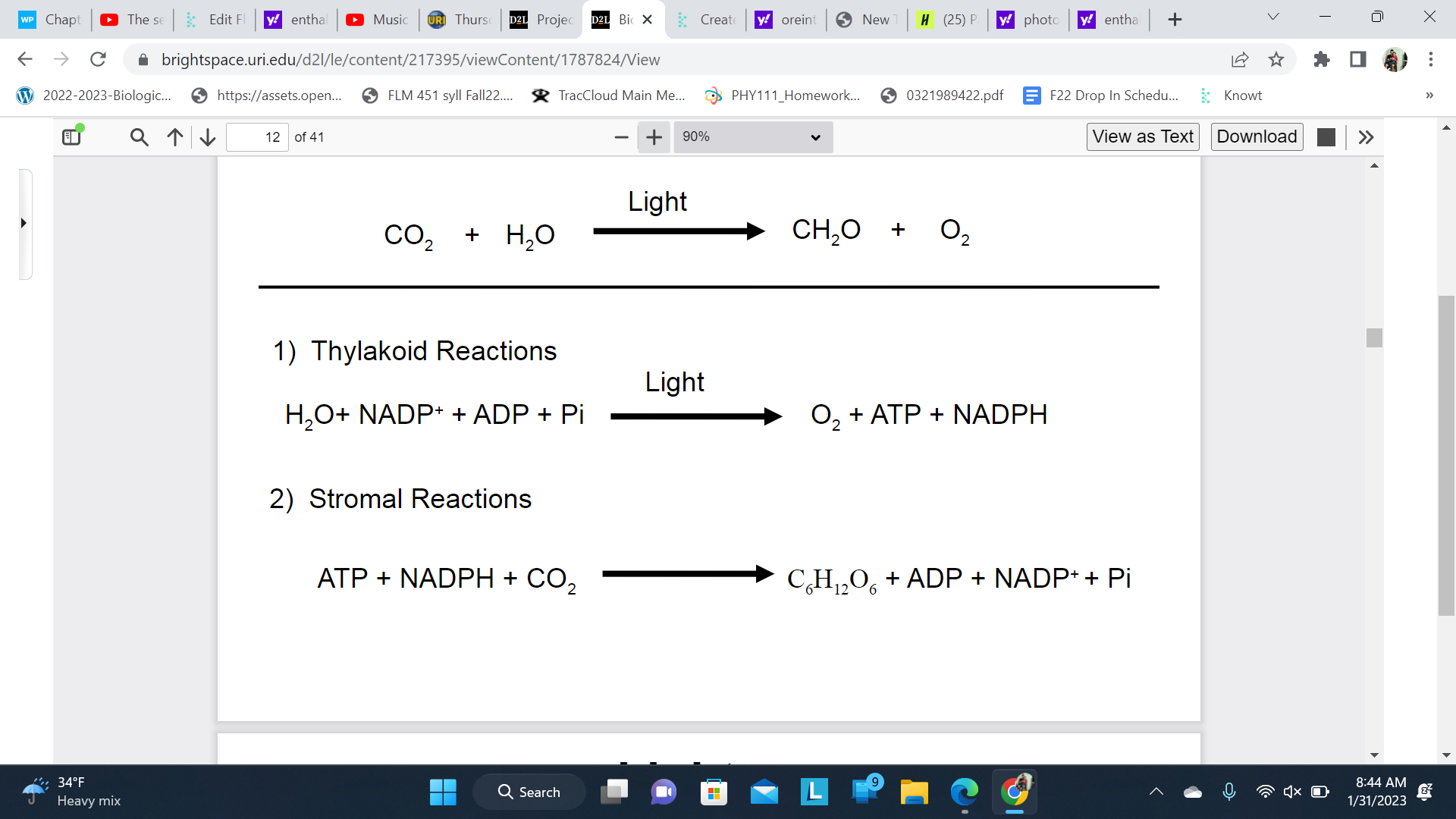
Photosynthesis can be divided into two phases
\
12
New cards
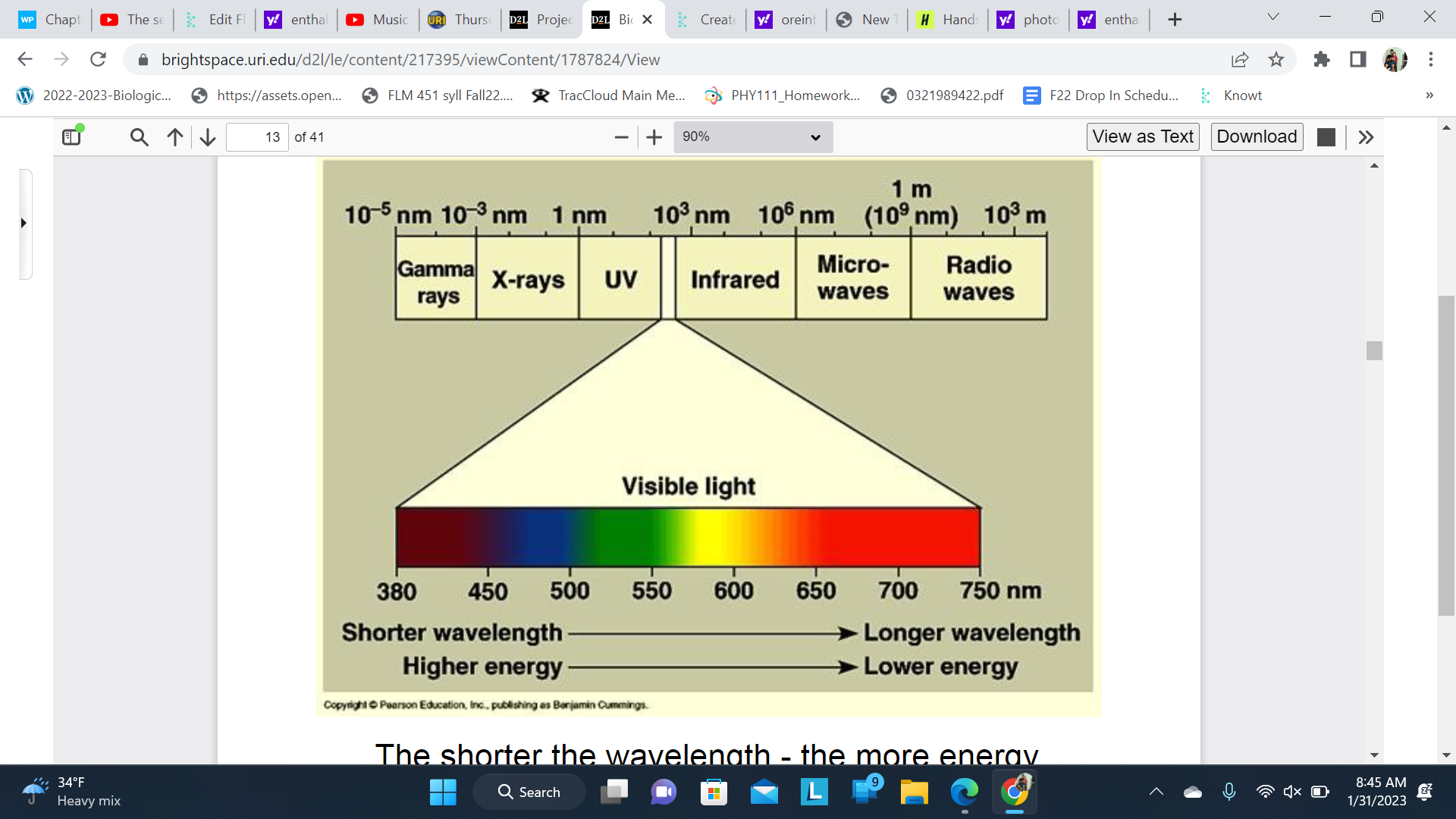
Light
The shorter the wavelength - the more energy
\
Violent on the left has the violent
\
Violent on the left has the violent
13
New cards
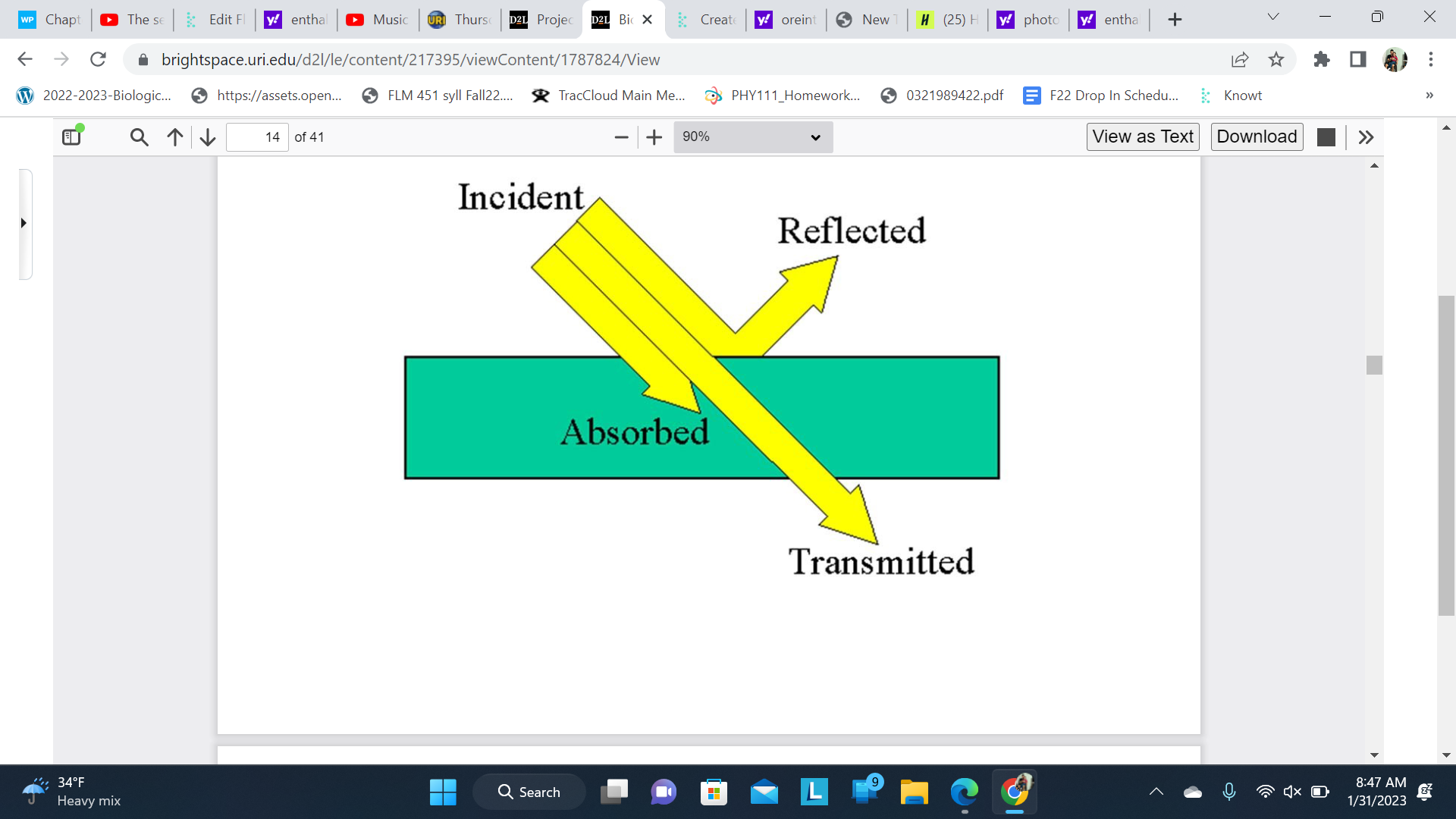
Light interactions with matter
Plants eat light
14
New cards
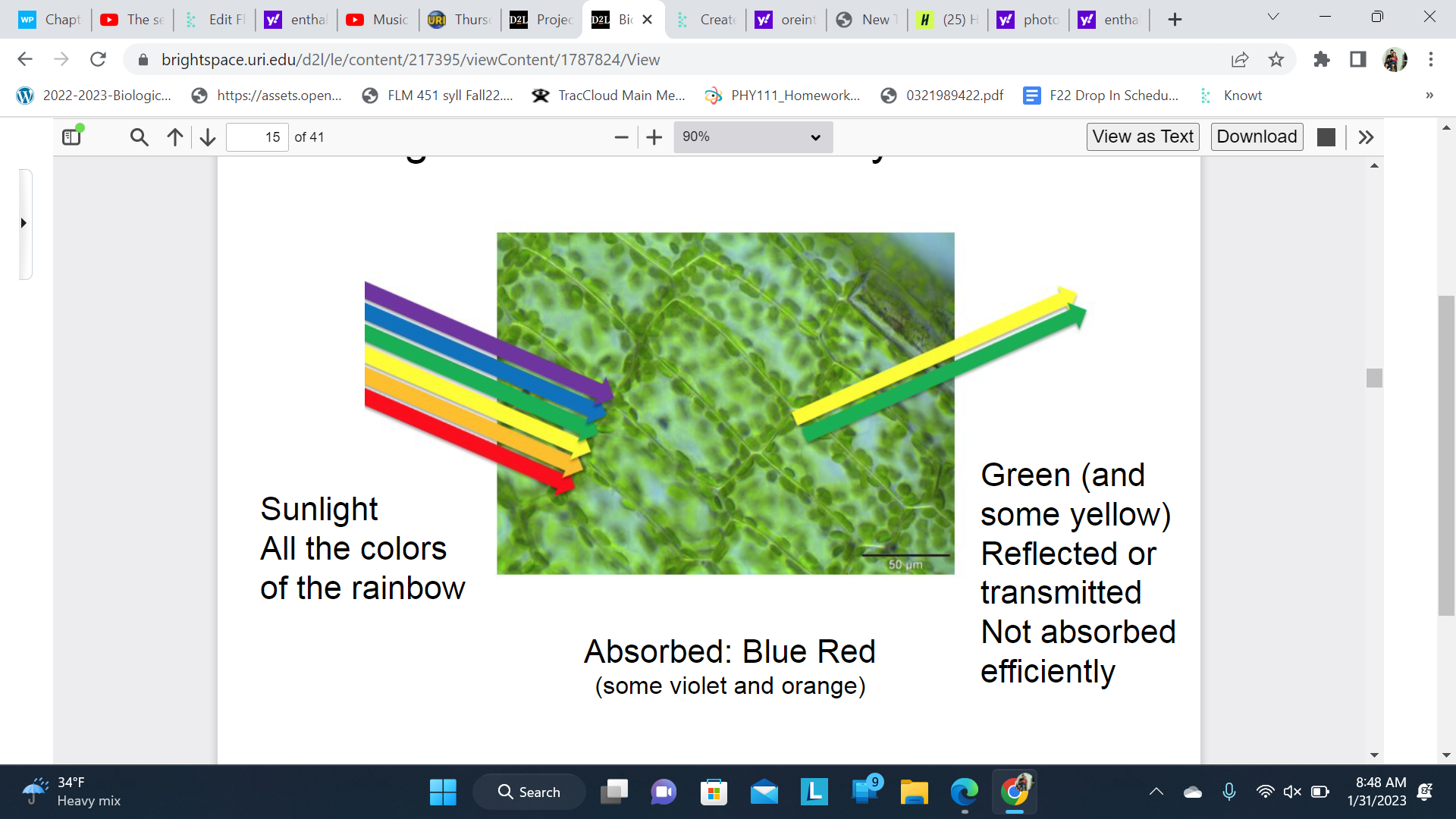
Light absorbed for Photosynthesis
Red and Blue are absorbed
\
Some violent and orange is absorbed
\
Green and some yellow reflected is reflected and not absorbed
\
The plant appears green because green light is reflected instead of absorbed.
\
Some violent and orange is absorbed
\
Green and some yellow reflected is reflected and not absorbed
\
The plant appears green because green light is reflected instead of absorbed.
15
New cards
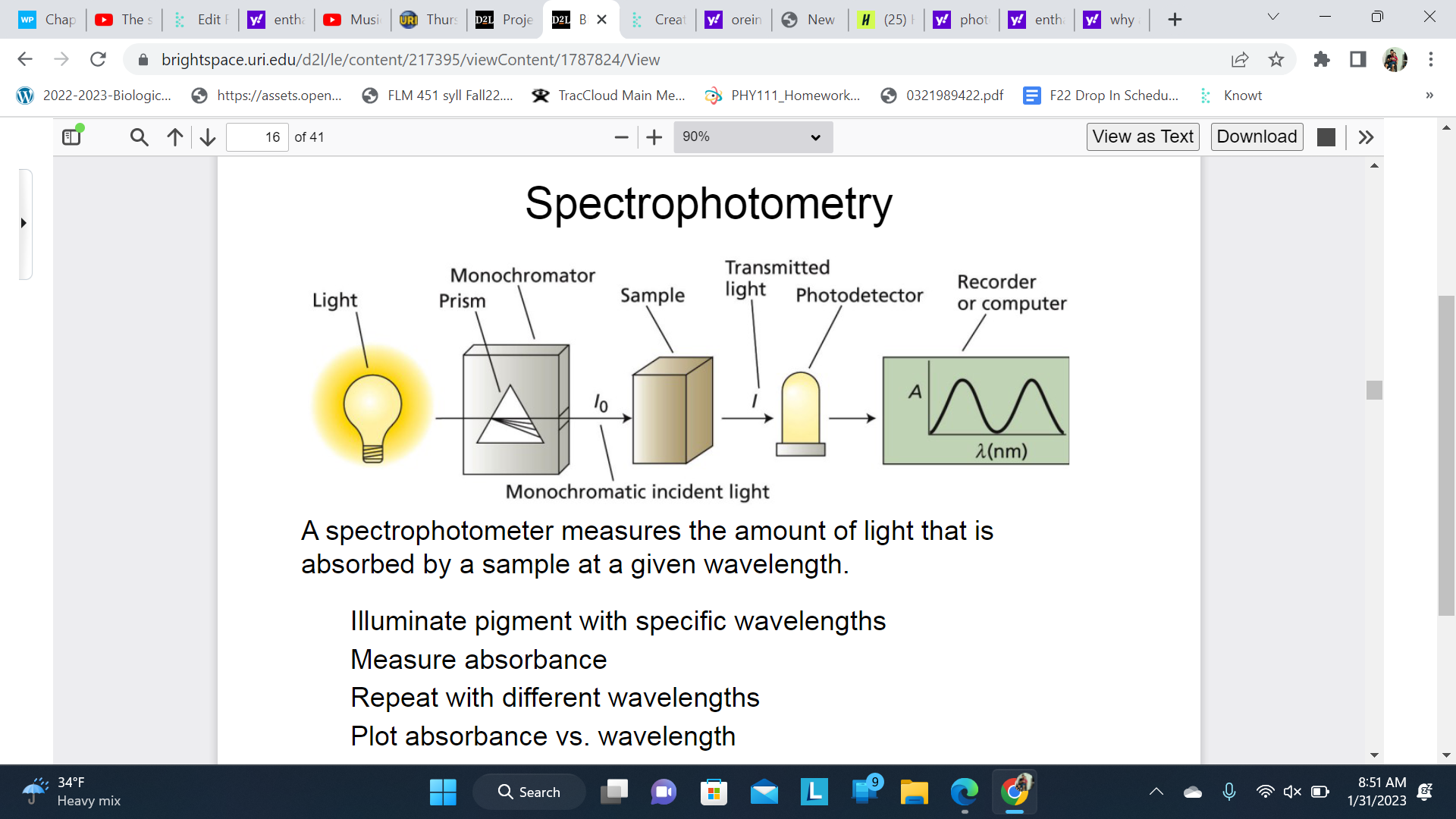
Spectrophotometry
A spectrophotometer measures the amount of light that is absorbed by a sample at a given wavelength.
\
Illuminate pigment with specific wavelengths
Measure absorbance
Repeat with different wavelengths
Plot absorbance vs. wavelength
\
Each specific pigment has a spectrographic ‘fingerprint’
\
Illuminate pigment with specific wavelengths
Measure absorbance
Repeat with different wavelengths
Plot absorbance vs. wavelength
\
Each specific pigment has a spectrographic ‘fingerprint’
16
New cards
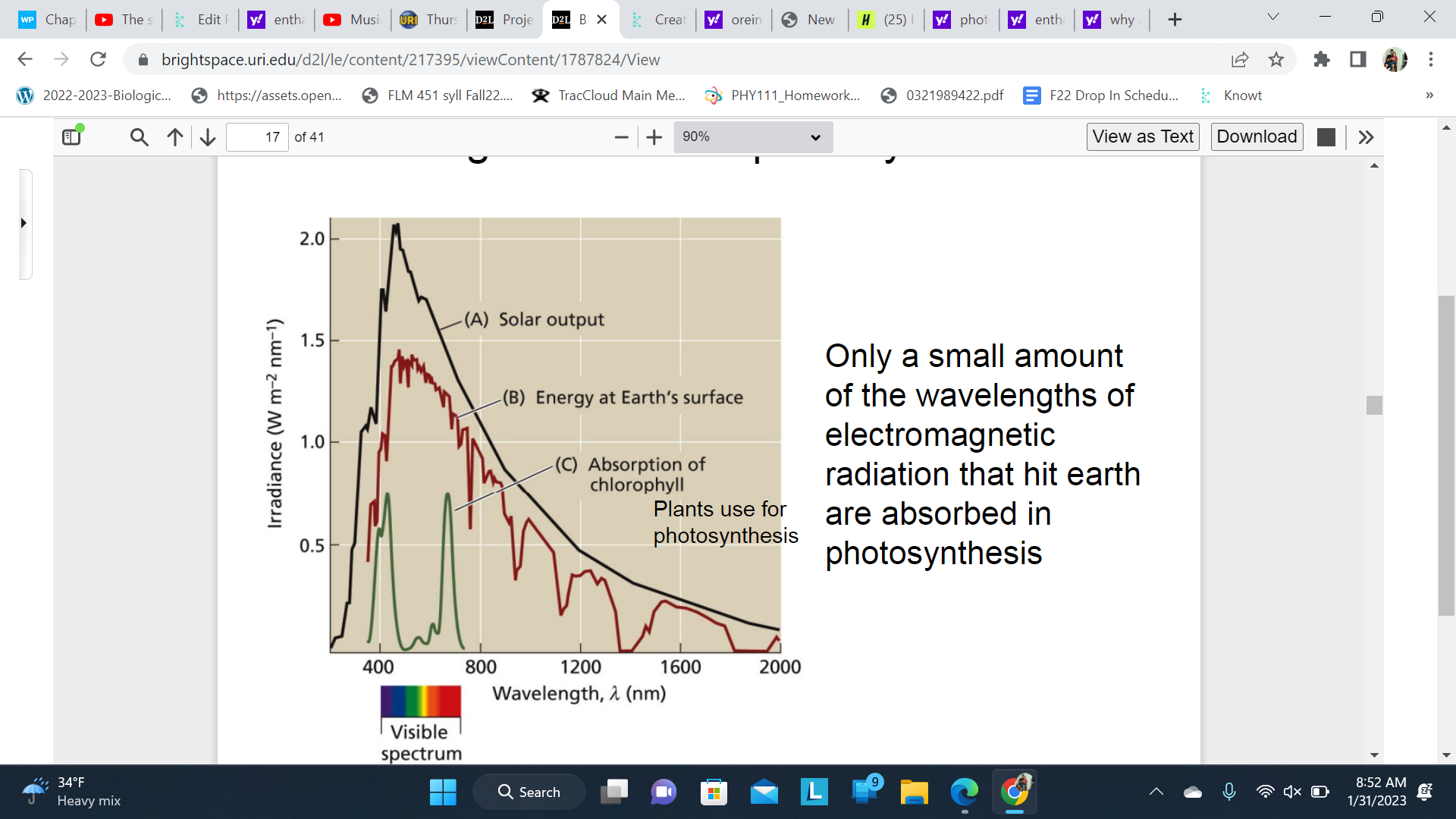
What light is used for photosynthesis?
Only a small amount of the wavelengths of electromagnetic radiation that hit earth are absorbed in photosynthesis
17
New cards
Common pigments of plants
Pigment means a colored compound
\
Pigment are compound that absorb light
\
1. Chlorophyll(a and b)
2. Carotenoids and Xanthophylls(carrots)
3. Phycobillins(in algae)
\
Pigment are compound that absorb light
\
1. Chlorophyll(a and b)
2. Carotenoids and Xanthophylls(carrots)
3. Phycobillins(in algae)
18
New cards
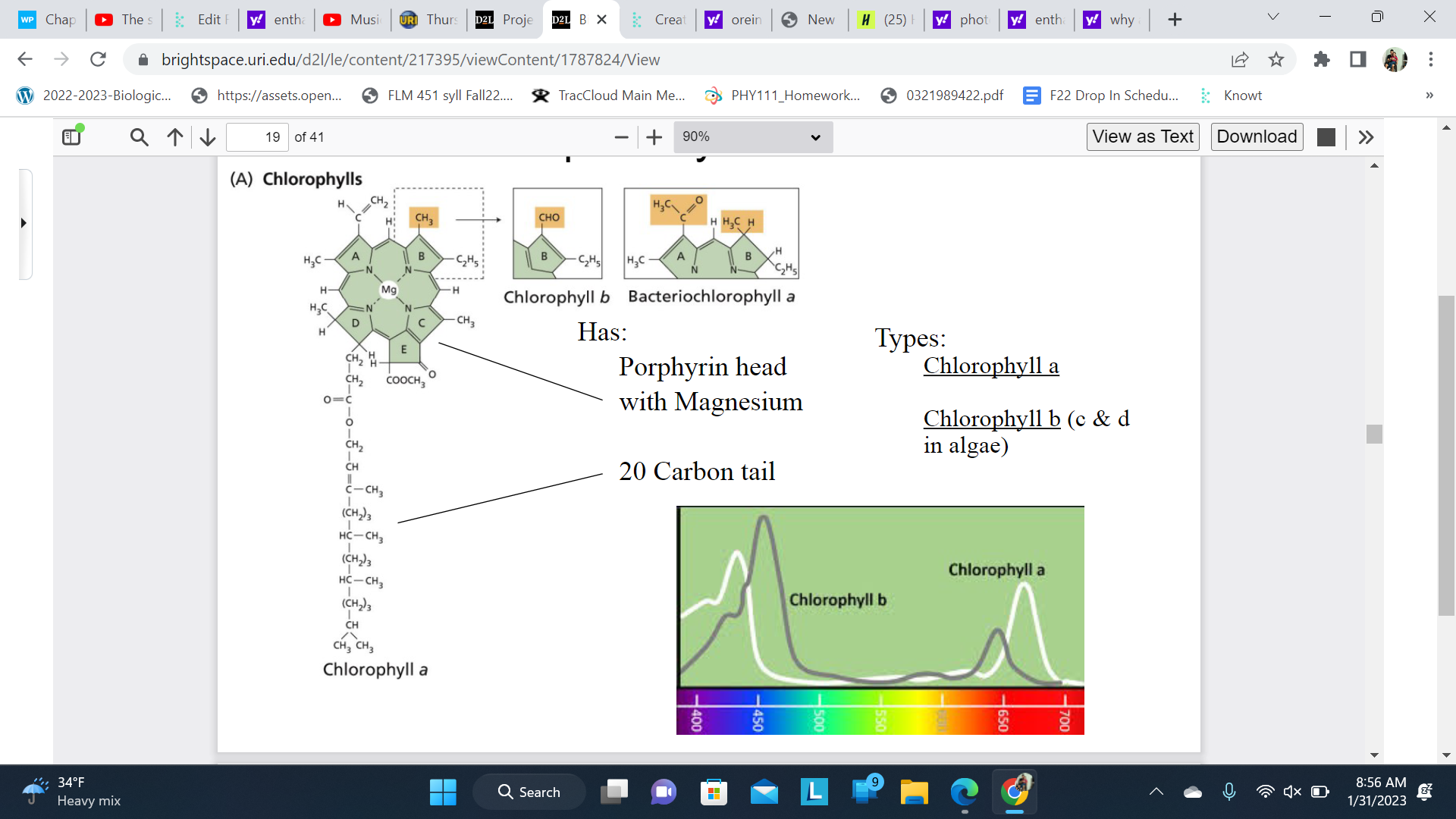
Chlorophylls
Large chlorophylls ring
\
A glove or mit to absorb the light
\
Chlorophyll a is the primary pigment inphotosynthesis
\
A glove or mit to absorb the light
\
Chlorophyll a is the primary pigment inphotosynthesis
19
New cards
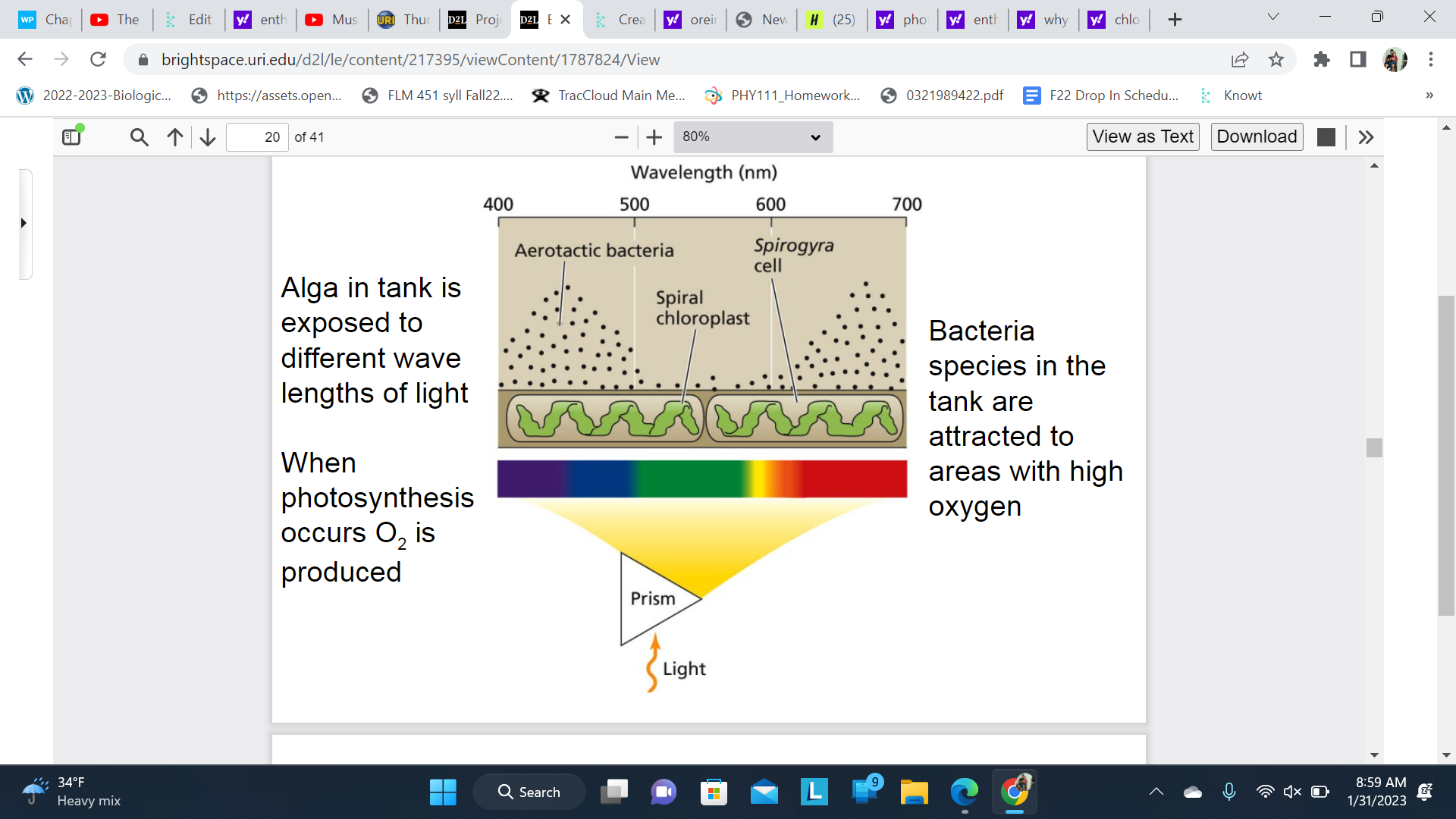
The first action spectum
places that had more photosynthesis produce more oxygen
20
New cards
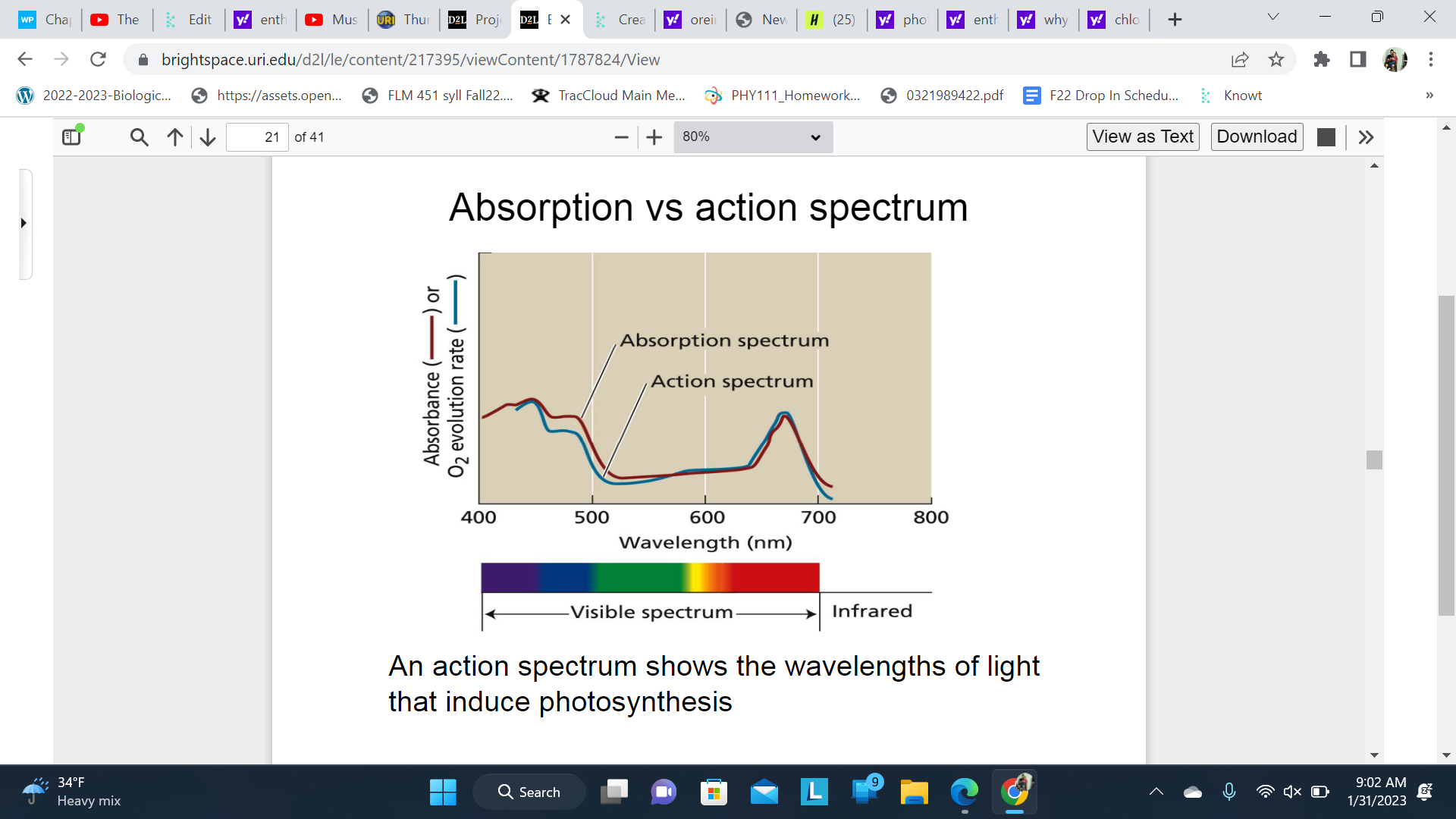
Absorption vs action spectrum
Since both spectrums match this proves Chlorophyll is the primary pigment that feeds photosynthesis
\
* An absorption spectrum shows all the light typically absorbed by a leaf. An action spectrum, meanwhile, shows all the light that is actually used for photosynthesis. The similarity of the action spectrum of photosynthesis and the absorption spectrum of chlorophyll tells us that chlorophyll is the most important pigments in the process.
\
* An absorption spectrum shows all the light typically absorbed by a leaf. An action spectrum, meanwhile, shows all the light that is actually used for photosynthesis. The similarity of the action spectrum of photosynthesis and the absorption spectrum of chlorophyll tells us that chlorophyll is the most important pigments in the process.
21
New cards
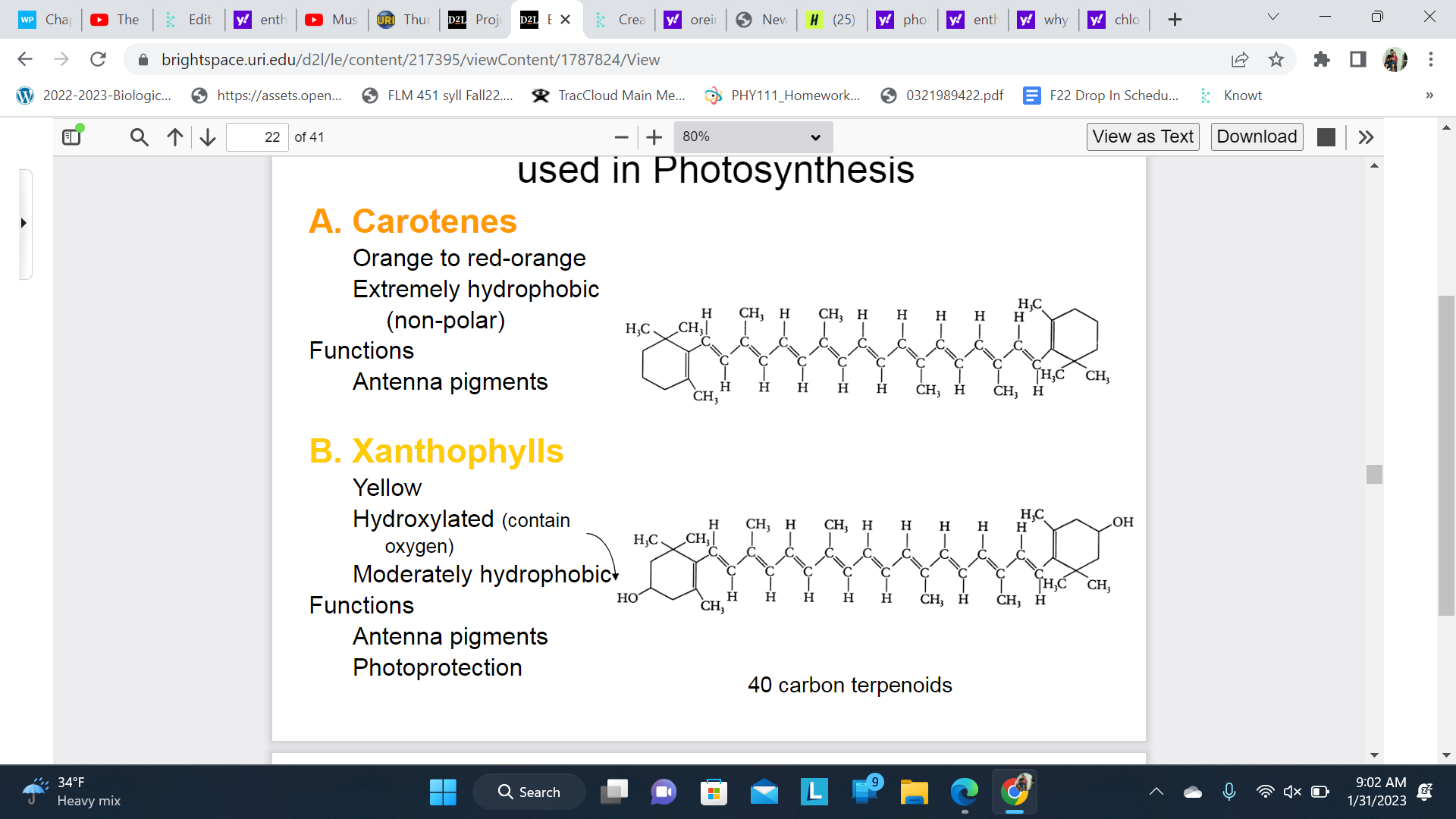
Carotenoid also absorb light used in Photosynthesis
A. Carotenes
Orange to red-orange
Extremely hydrophobic (non-polar)
Functions: Antenna pigments
\
B. Xanthophylls
Yellow
Hydroxylated (contain oxygen)
Moderately hydrophobic
Functions: Antenna pigments
Photoprotection
\
The role of the antenna pigments is to collect light energy from the sun and transfer it to reaction centers.
Orange to red-orange
Extremely hydrophobic (non-polar)
Functions: Antenna pigments
\
B. Xanthophylls
Yellow
Hydroxylated (contain oxygen)
Moderately hydrophobic
Functions: Antenna pigments
Photoprotection
\
The role of the antenna pigments is to collect light energy from the sun and transfer it to reaction centers.
22
New cards
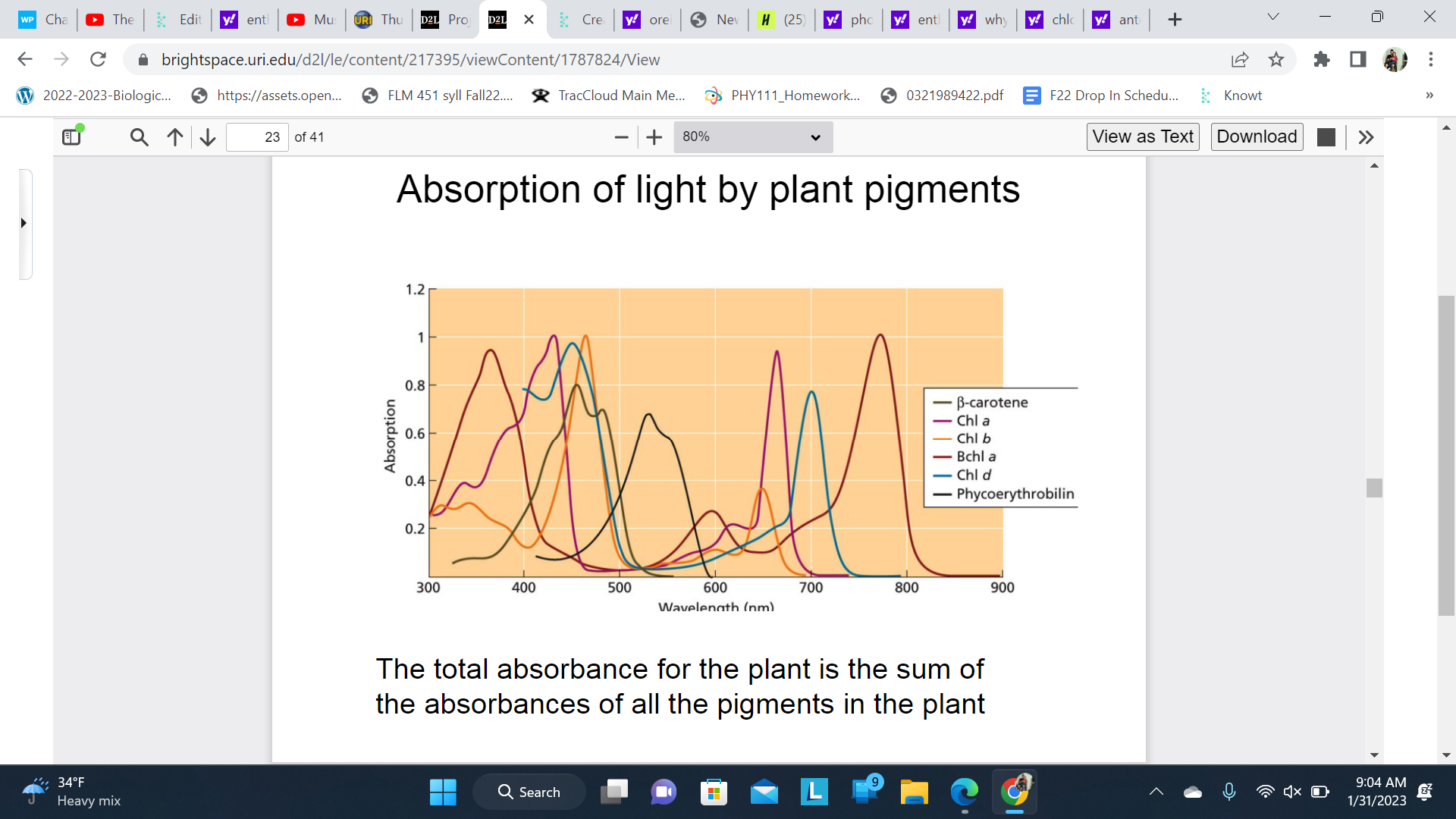
Absorption of light by plant pigments
The total absorbance for the plant is the sum of the absorbances of all the pigments in the plant
23
New cards
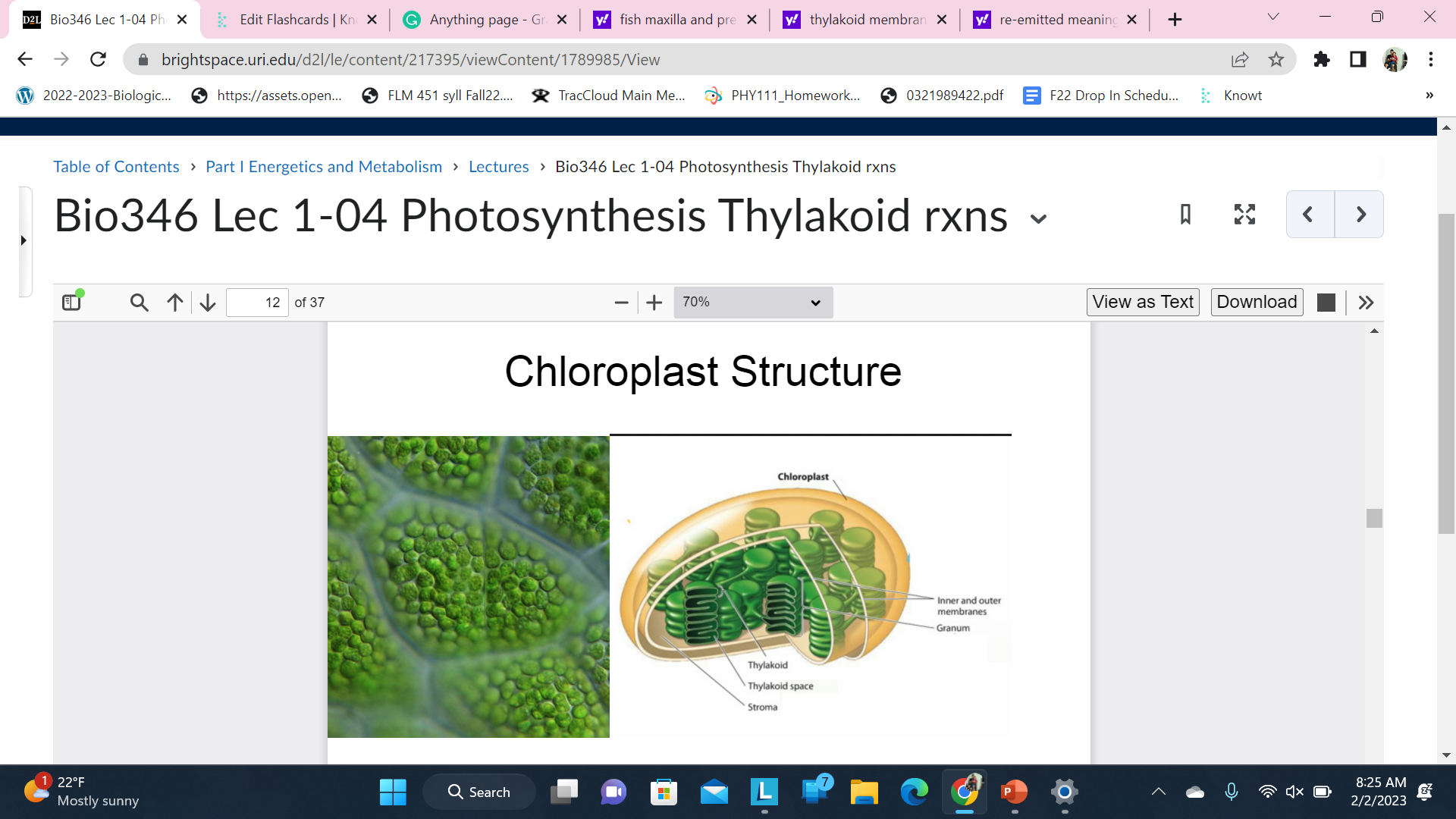
Chloroplast structure
Leaf Cells
\
Photosynthetic Pigments (Chlorophyll etc.) and Photosystems are located in the thylakoid membranes inside the chloroplast
\
remember stroma
\
thylakoid membrane should not be green they should be…
\
Photosynthetic Pigments (Chlorophyll etc.) and Photosystems are located in the thylakoid membranes inside the chloroplast
\
remember stroma
\
thylakoid membrane should not be green they should be…
24
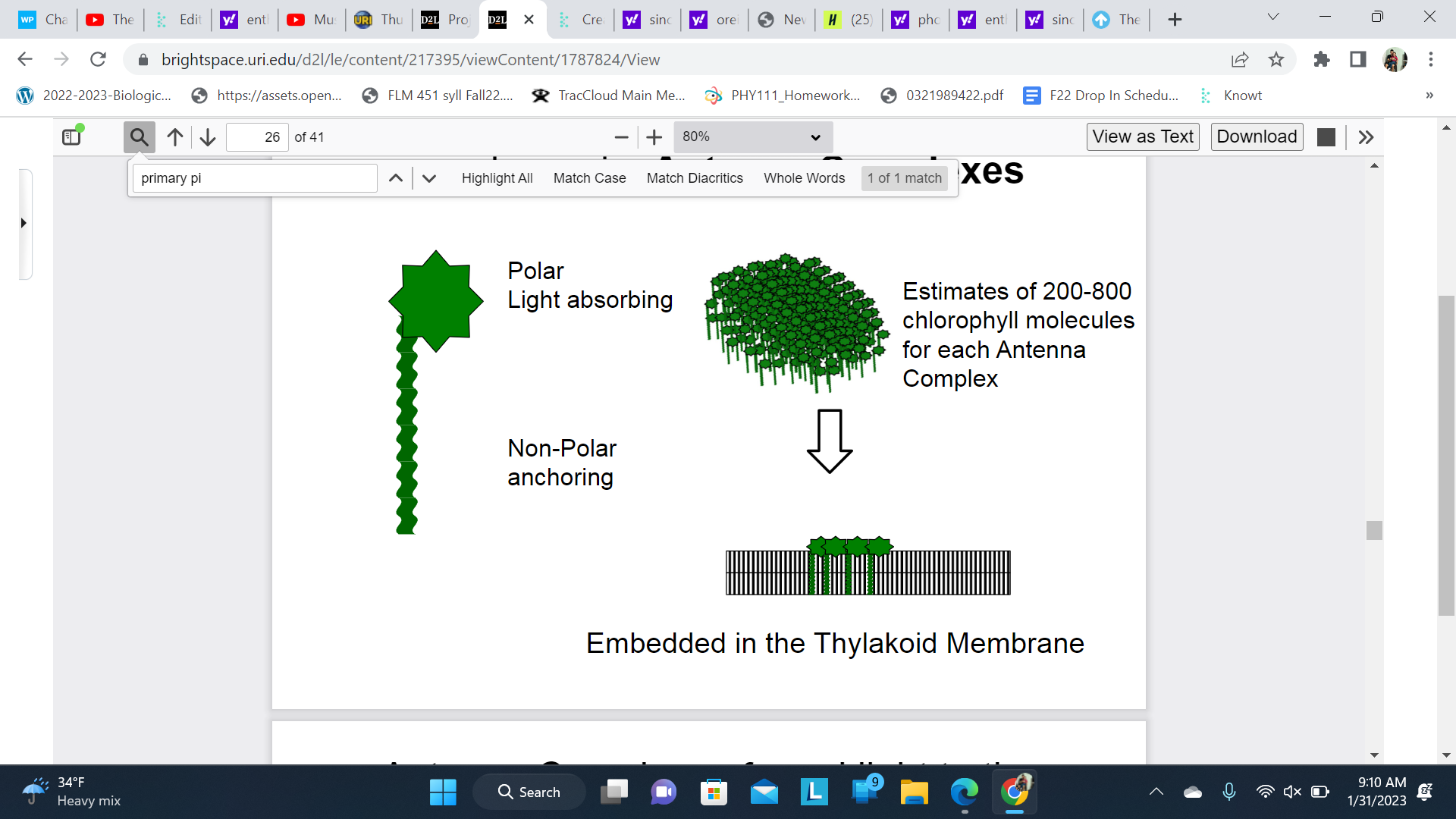
New cards
Chlorophyll Is anchored in the thylakoid membrane in Antenna Complexes
They can move laterally across the membrane
25
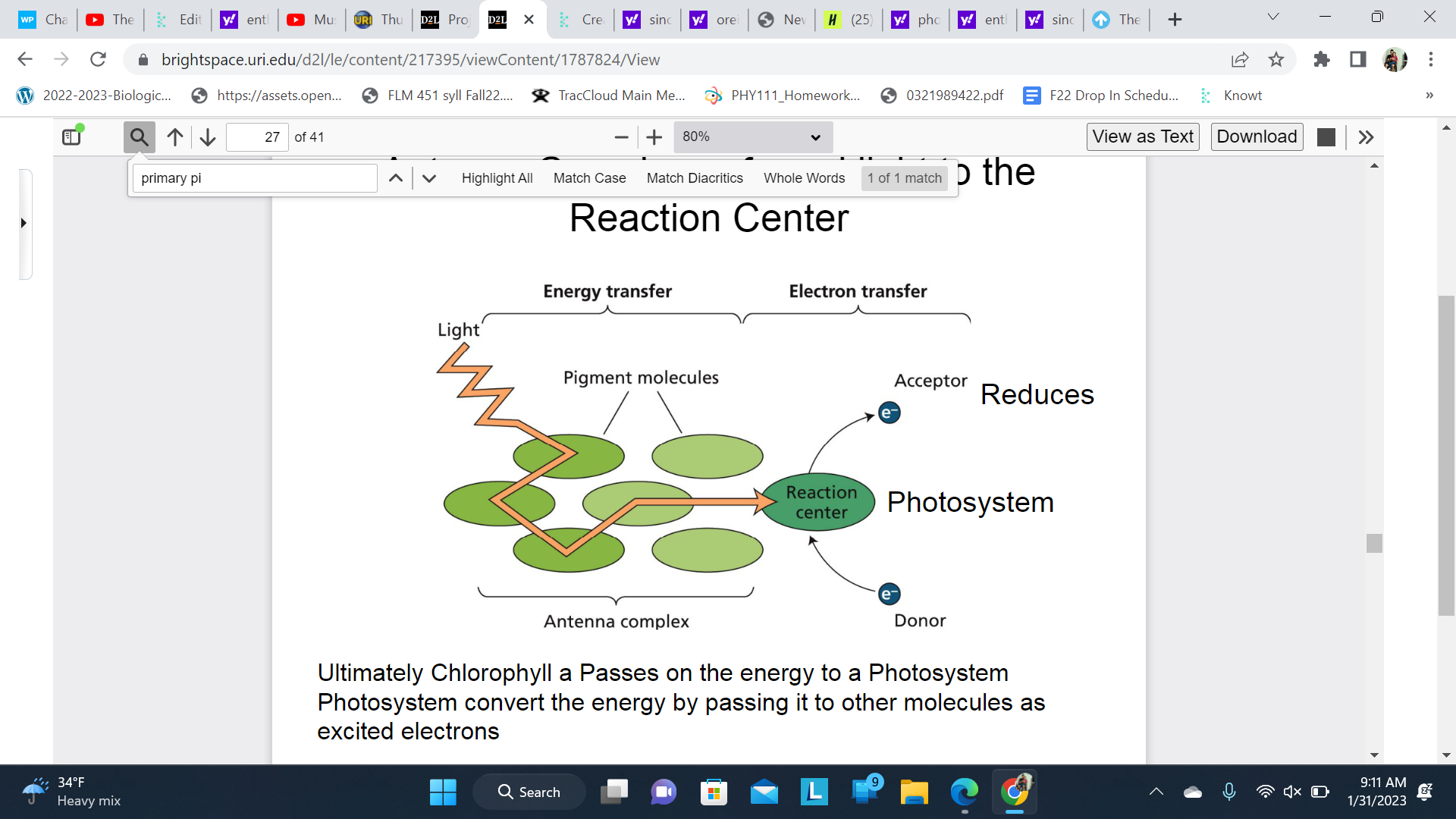
New cards
Antenna Complexes funnel light to the reaction center
Ultimately Chlorophyll a Passes on the energy to a Photosystem
\
Photosystem convert the energy by passing it to other molecules as excited electrons
\
Photosystem convert the energy by passing it to other molecules as excited electrons
26
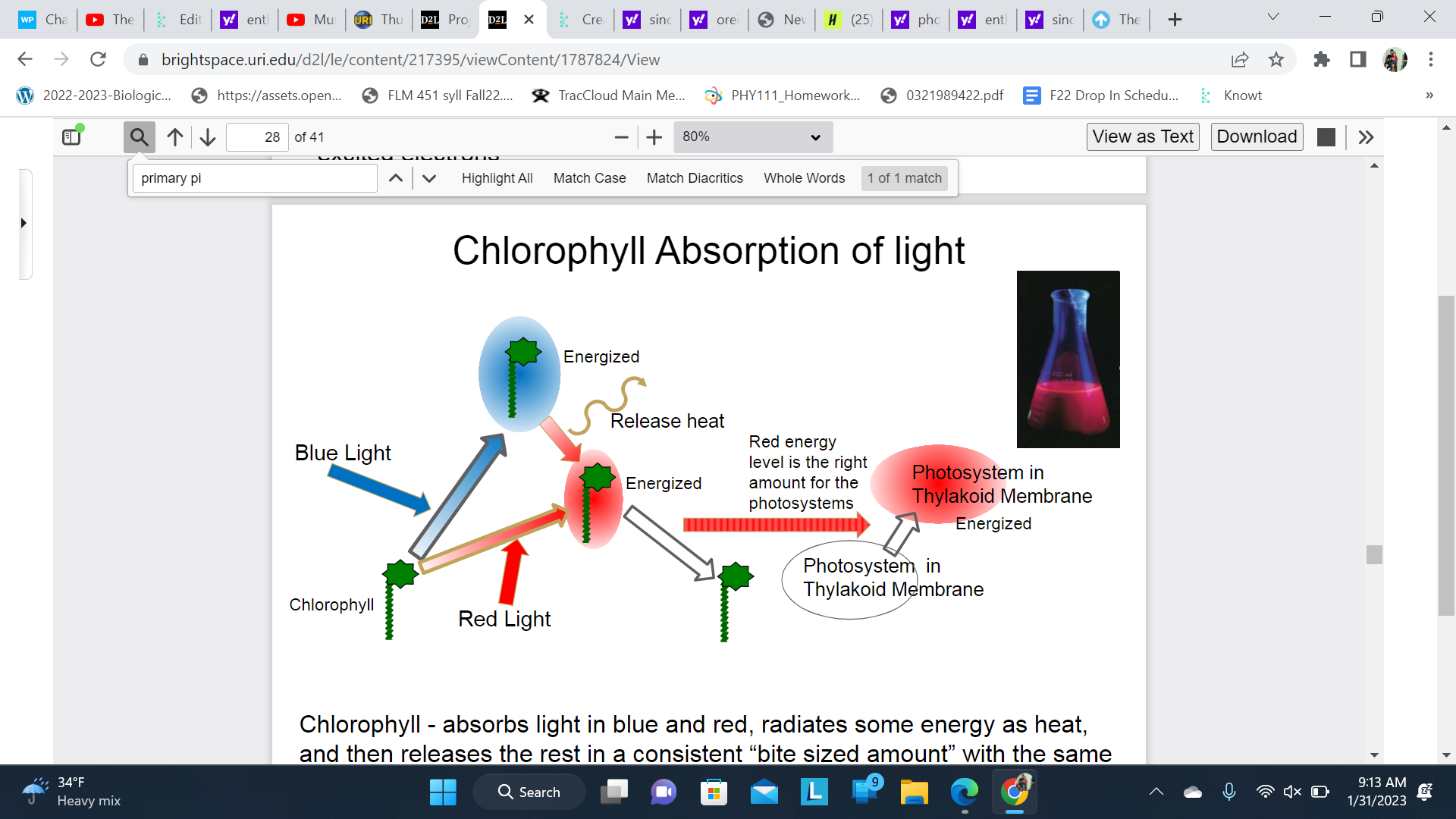
New cards
Chlorophyll Absorption of light
Chlorophyll - absorbs light in blue and red, radiates some energy as heat, and then releases the rest in a consistent “bite sized amount” with the same energy level as in red feeding the energy into photosynthetic reactions
If uncoupled chlorophyll will fluoresce red when exposed to blue or red light
\
Absorbs blue= has to much energy; has to release
\
Absorbs red= get bite sized energy
\
If uncoupled chlorophyll will fluoresce red when exposed to blue or red light
\
Absorbs blue= has to much energy; has to release
\
Absorbs red= get bite sized energy
\
27
New cards
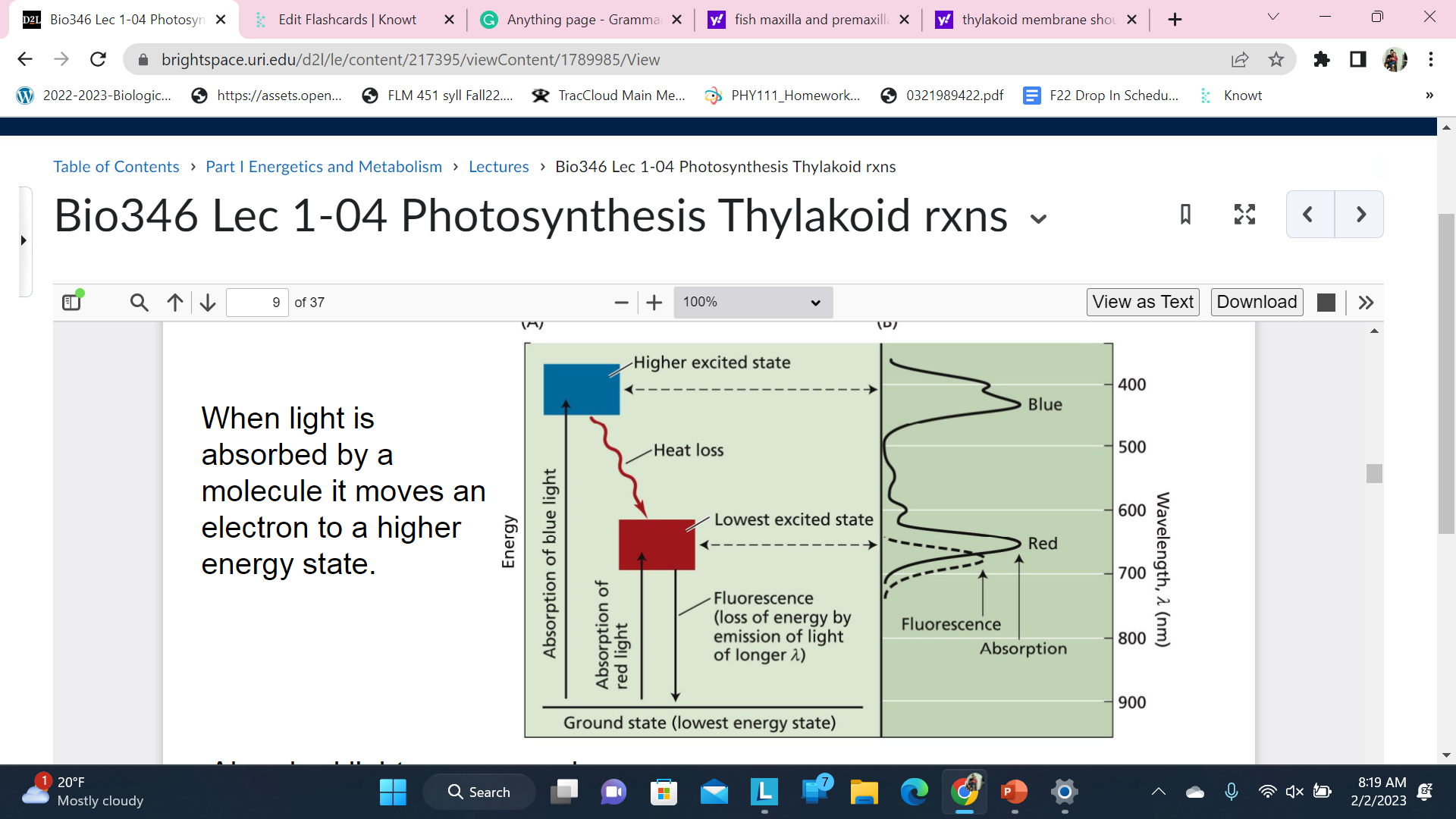
Whatt happen with absorbed light
When light is absorbed by a molecule it moves an electron to a higher energy state
\
Absorbed light energy can be:
radiated as heat
* re-emitted / fluoresced as light
* transferred to another molecule by resonance
used to reduce another compound (donates electrons)
\
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation.
\
Absorbed light energy can be:
radiated as heat
* re-emitted / fluoresced as light
* transferred to another molecule by resonance
used to reduce another compound (donates electrons)
\
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation.
28
New cards
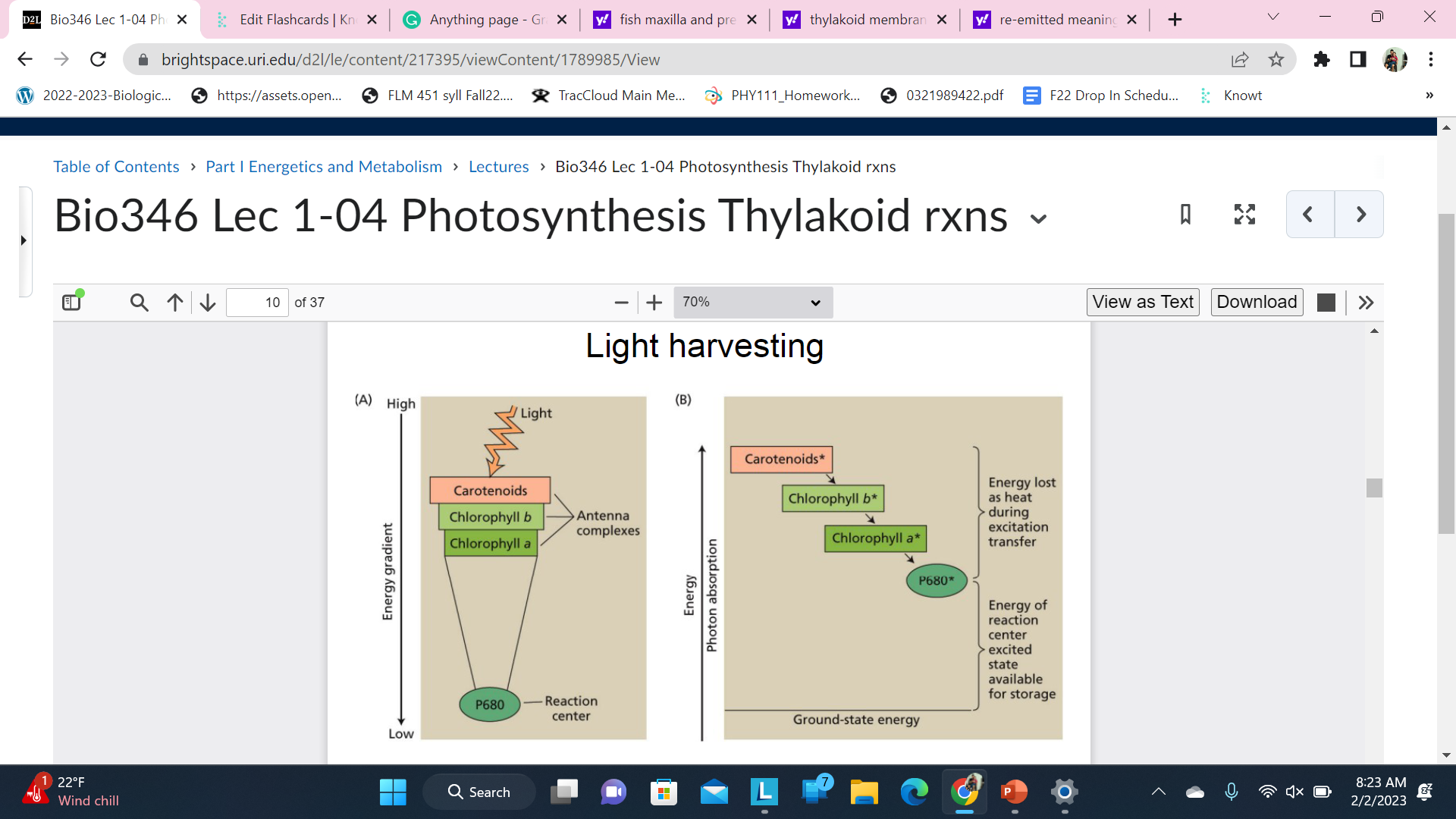
Light harvesting
Energy transfer in the antenna complex is random but, because energy is lost during some of the transfers, it funnels the light to the reaction center(p680 here)
29
New cards
Ask
What is the point of this slide
30
New cards
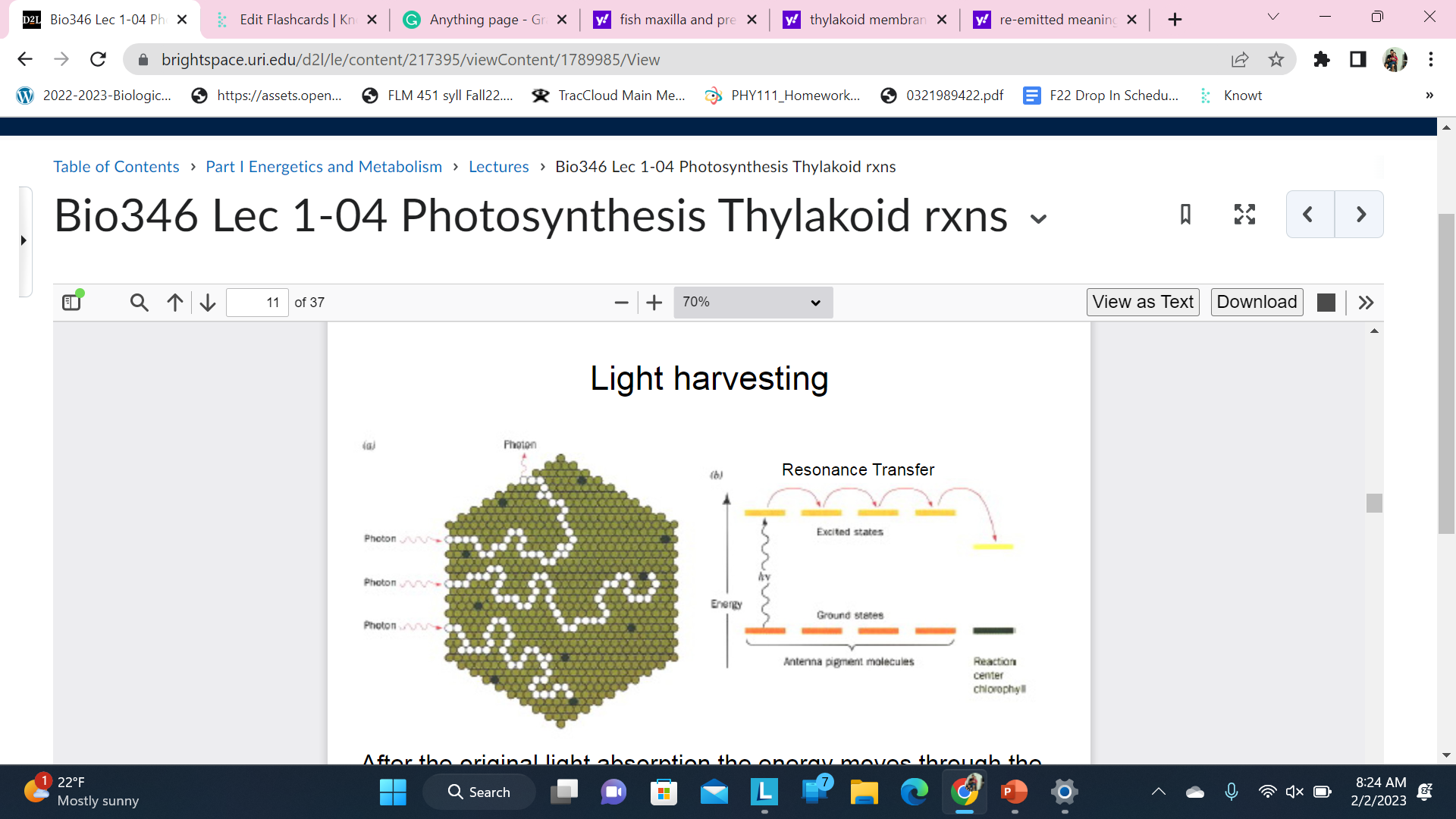
Light harvesting
After the original light absorption the energy moves through the chlorophyll and carotenoid molecules in the Antenna Complex by Resonance transfer. Transfers occur as fast as femto seconds
31
New cards
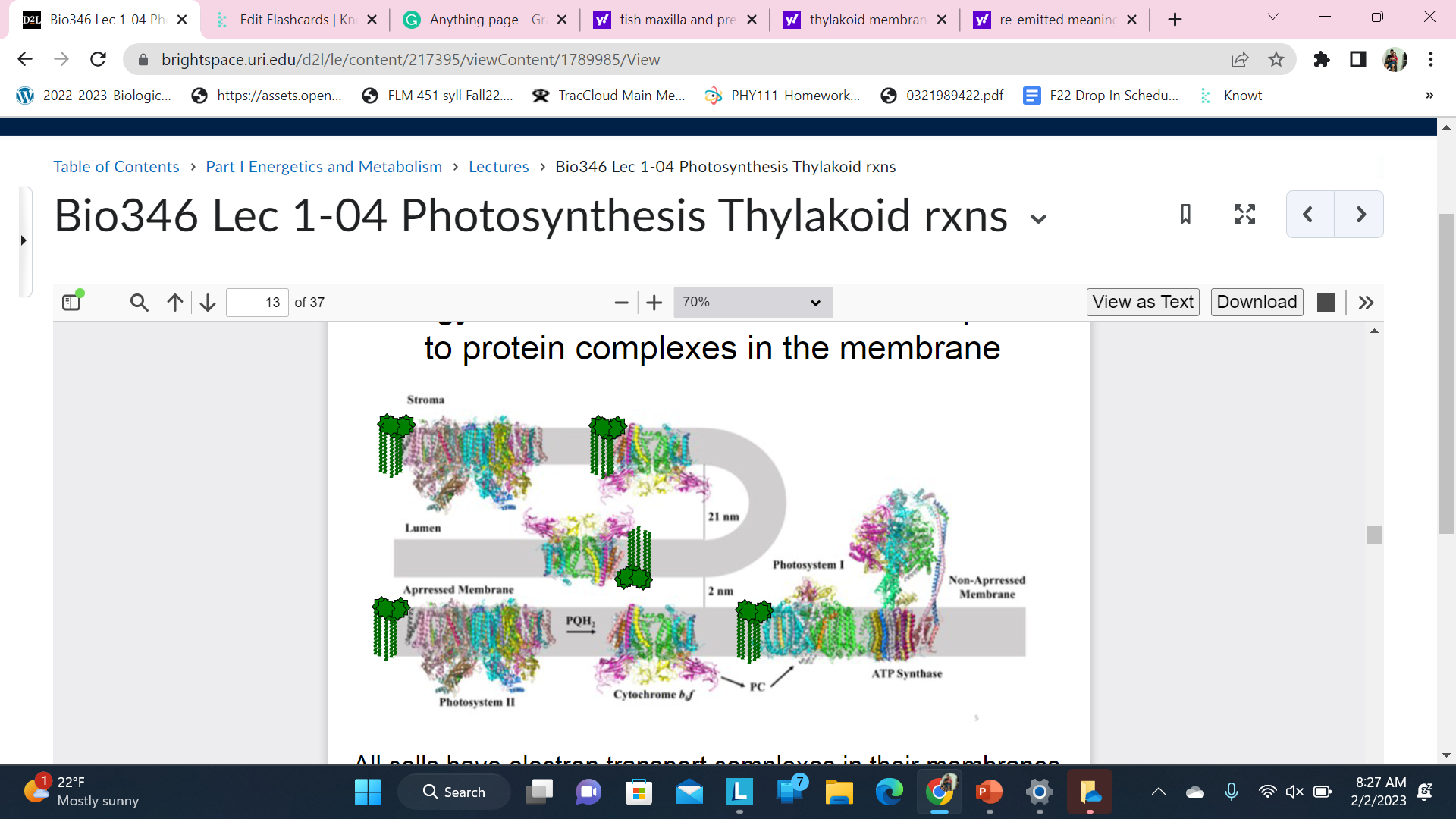
Energy is transferred from antenna complexes to protein complexes in the membrane.
All cells have electron transport complexes in their membranes
32
New cards
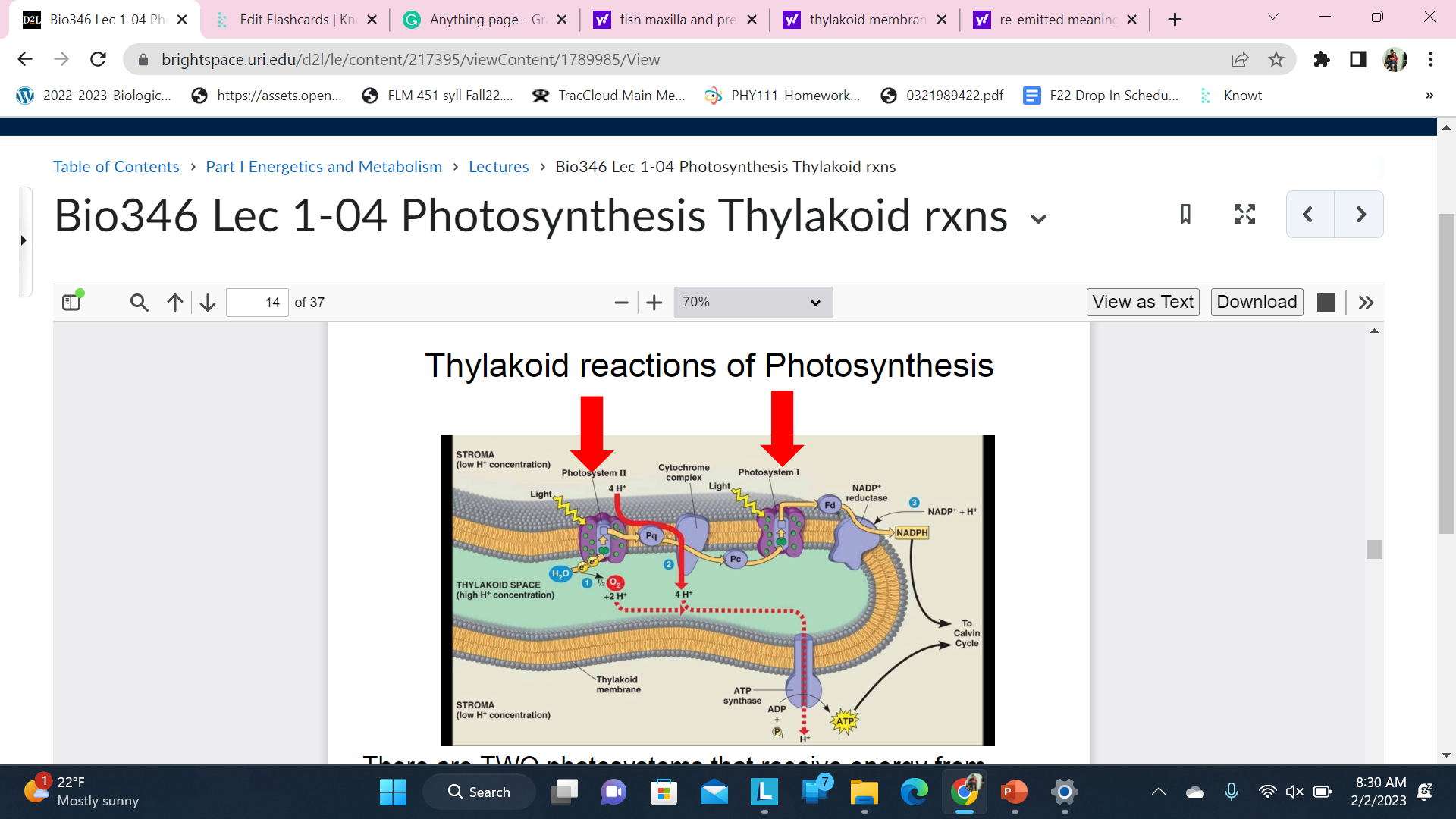
Thylakoid reactions of Photosynthesis
There are TWO photosystems that receive energy from \n chlorophyll/antenna complexes - \n
Photosystem II, PS II or p680 \n Photosystem I, PS I or p700
Photosystem II, PS II or p680 \n Photosystem I, PS I or p700
33
New cards
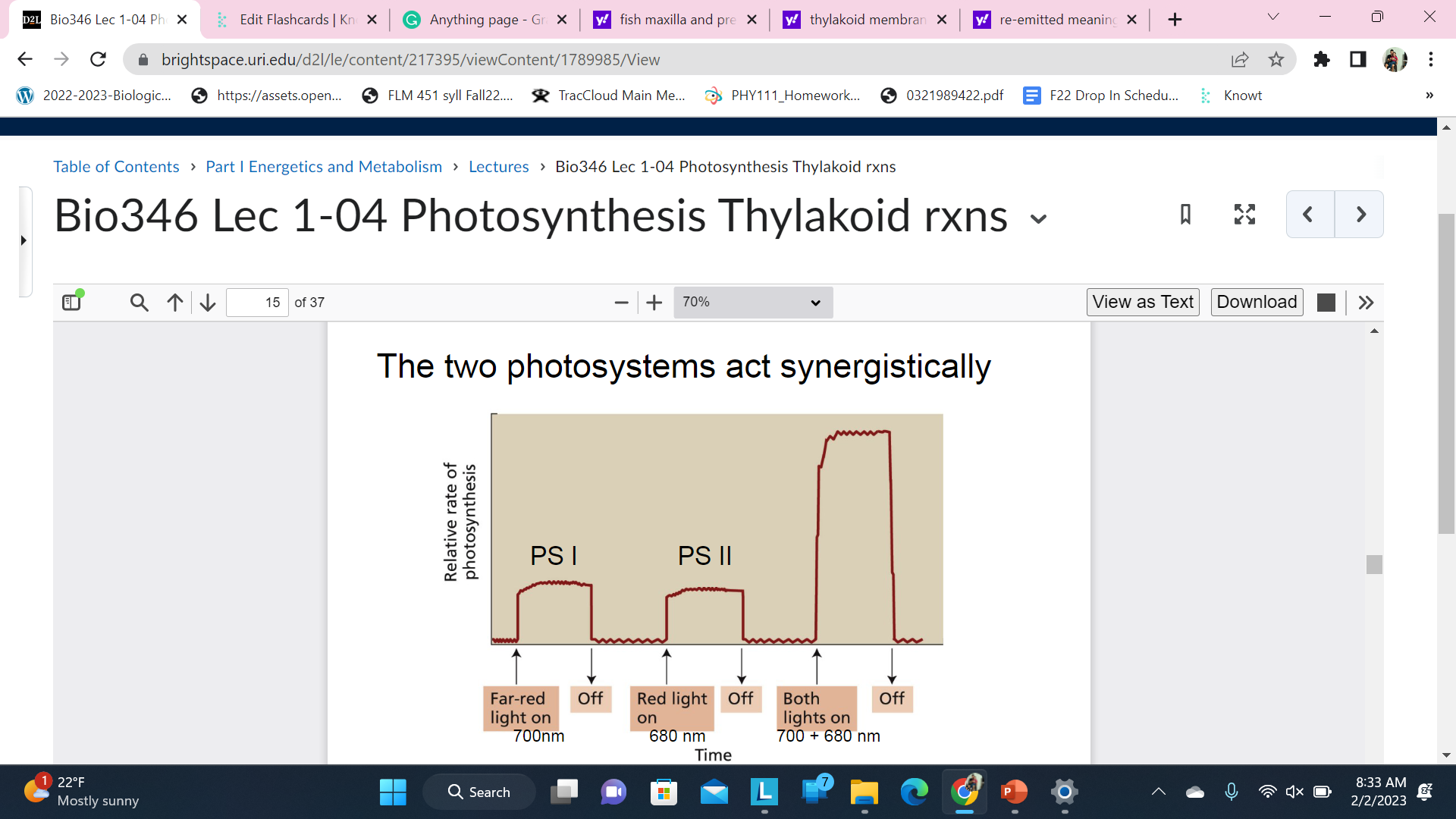
The two photosystems act synergistically
Each Photosystem can absorb light to power photosynthesis but when both are activated they work synergistically - More then just the addition of the two parts
34
New cards
Energy + Electrons are required for photosynthesis
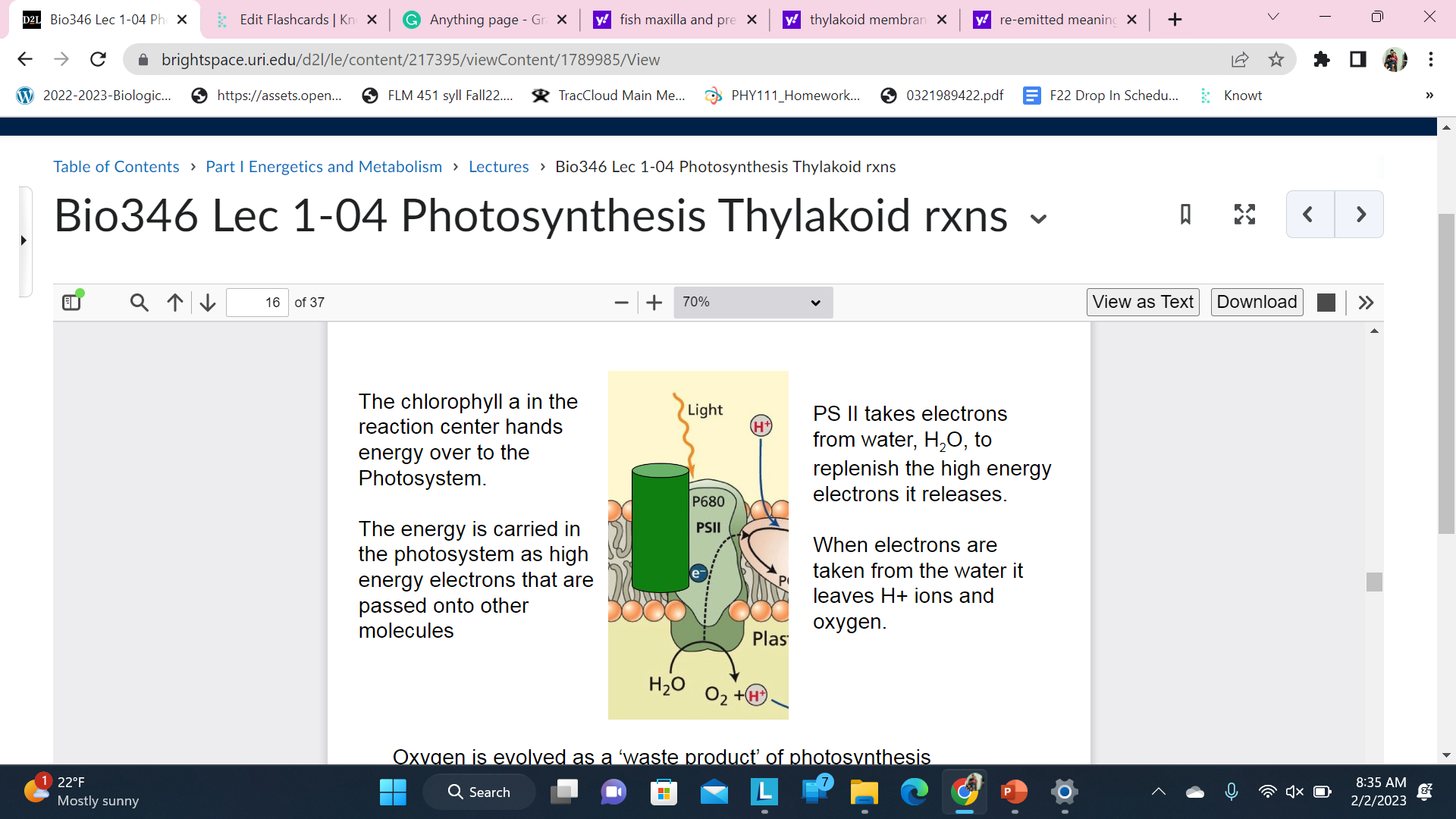
The chlorophyll a in the reaction center hands energy over to the Photosystem.
\
The energy is carried in the photosystem as high energy electrons that are passed onto other molecules
\
PS II takes electrons from water, H2O, to replenish the high energy electrons it releases.
\
When electrons are taken from the water it leaves H+ ions and oxygen
\
Oxygen is evolved as a ‘waste product’ of photosynthesis
\
The purpose of this slide: That oxygen is a waste product of photosynthesis(thylakoid reaction). **Energy + Electrons are required**
\
Oxygen becomes a free radical. Free radicals are bad because they are reactive and can cause cell damage.
\
The energy is carried in the photosystem as high energy electrons that are passed onto other molecules
\
PS II takes electrons from water, H2O, to replenish the high energy electrons it releases.
\
When electrons are taken from the water it leaves H+ ions and oxygen
\
Oxygen is evolved as a ‘waste product’ of photosynthesis
\
The purpose of this slide: That oxygen is a waste product of photosynthesis(thylakoid reaction). **Energy + Electrons are required**
\
Oxygen becomes a free radical. Free radicals are bad because they are reactive and can cause cell damage.
35
New cards
Cellular energy review
Cells are constantly using energy to maintain organixation
\
Reduced:
is gaining energy as high energy electrons
\
Reduced:
is gaining energy as high energy electrons
36
New cards
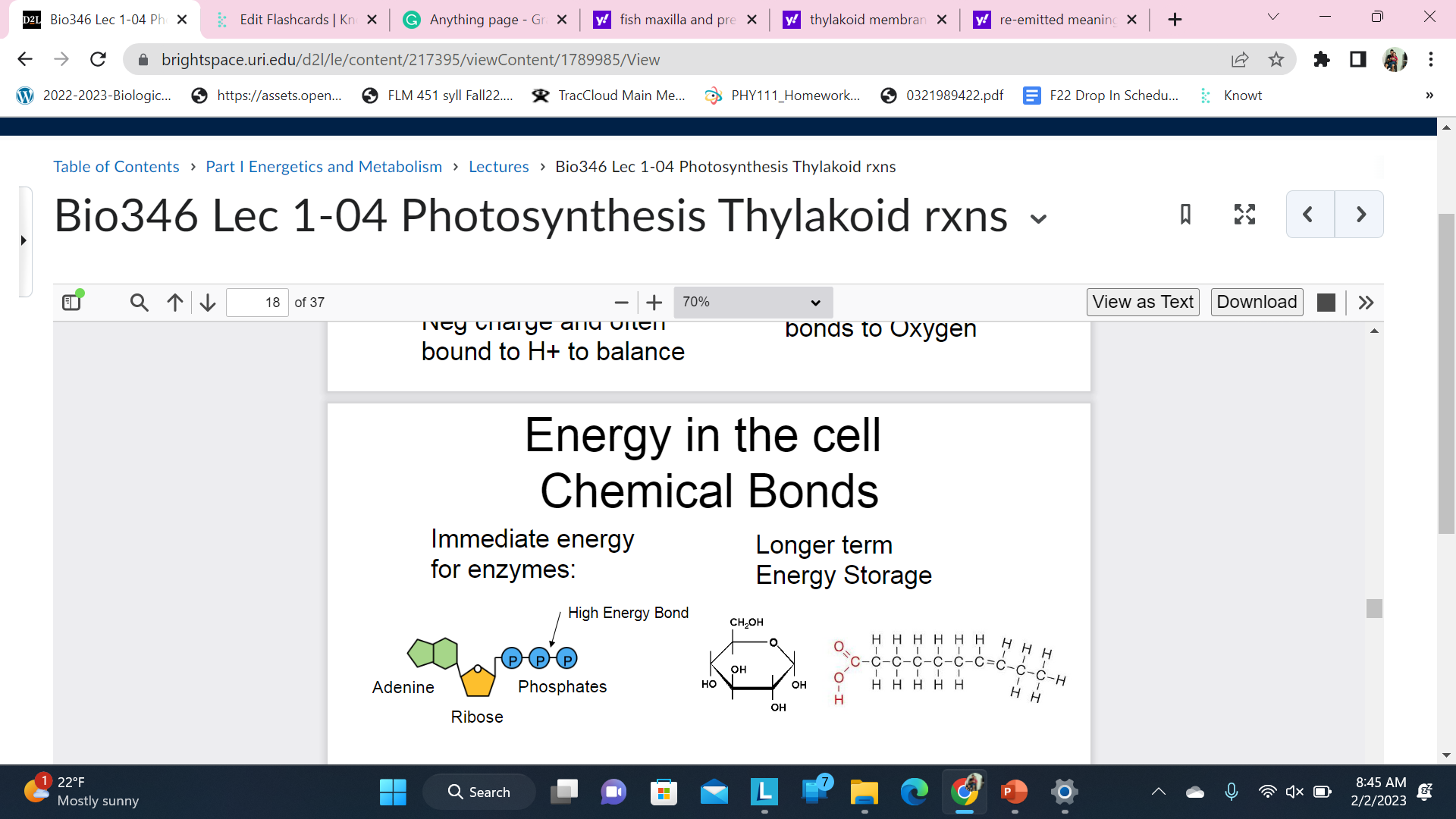
Energy in the cell \n Chemical Bonds
ATP is directly used to power enzymes but not safe to store in large quantities due to it reactivity
\
Carbohydrates and lips contain lots of energy in their bonds but not directly used to power enzyme
\
Carbohydrates and lips contain lots of energy in their bonds but not directly used to power enzyme
37
New cards
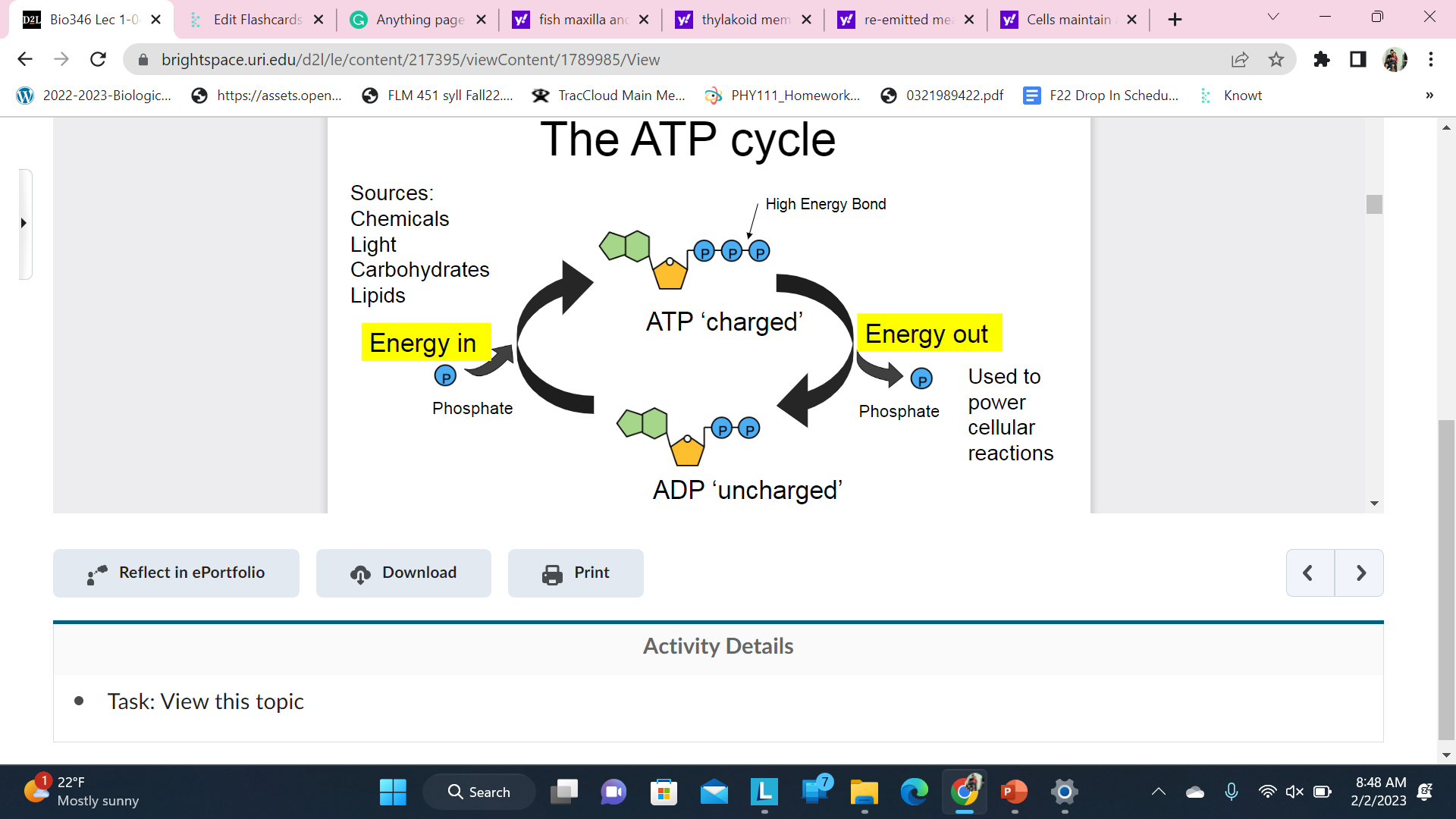
The ATP cycle
atp adenosine triphosphate the primary energy carrier of life
\
Cells maintain a constant level of ATP in the cytoplasm
\
Cells maintain a constant level of ATP in the cytoplasm
38
New cards
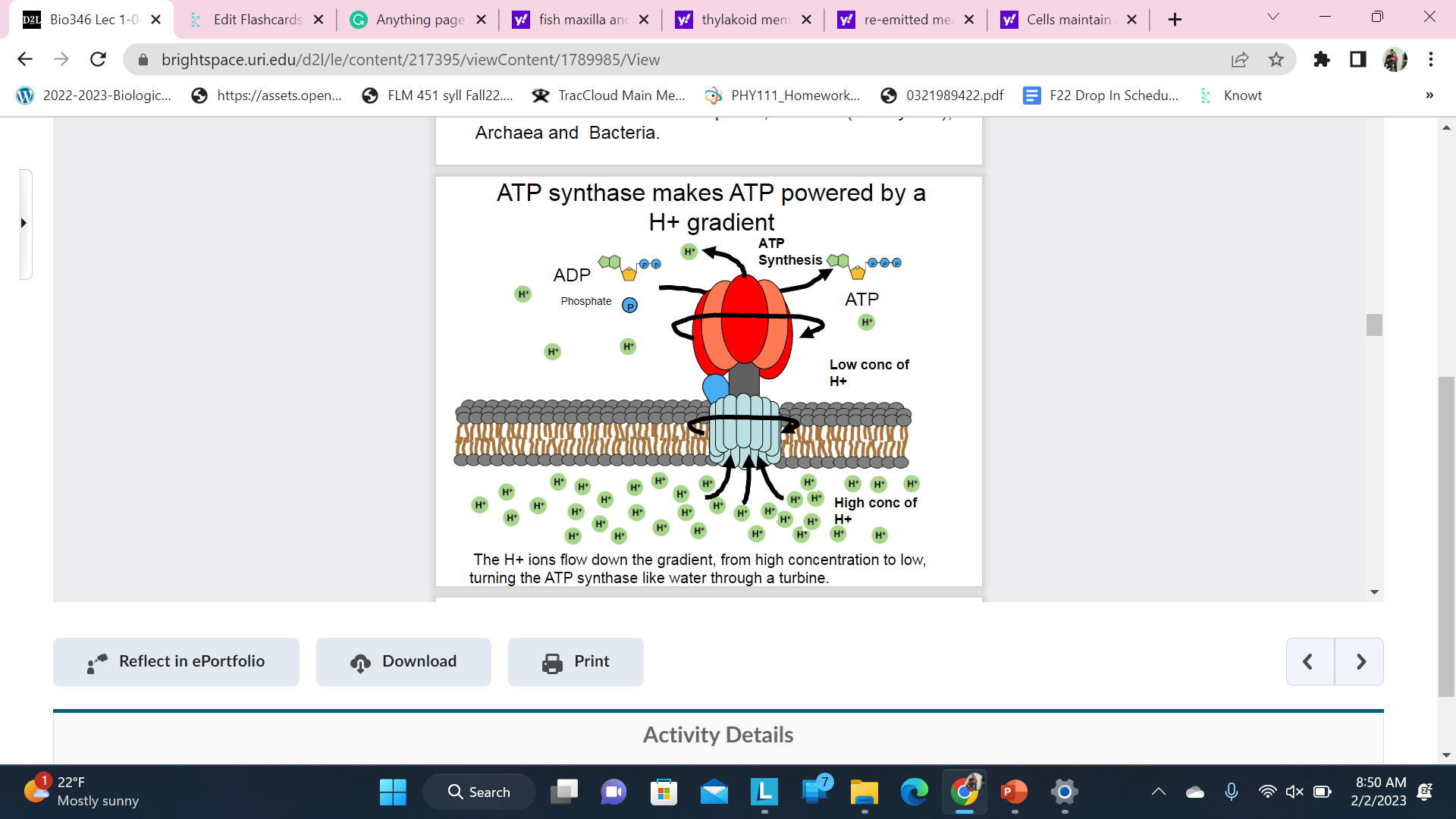
The enzyme that makes (most) ATP is called ATP Synthase
There are versions of this ancient membrane bound molecular machine in all life - plants, animals (eukaryotes), Archaea and Bacteria.
39
New cards
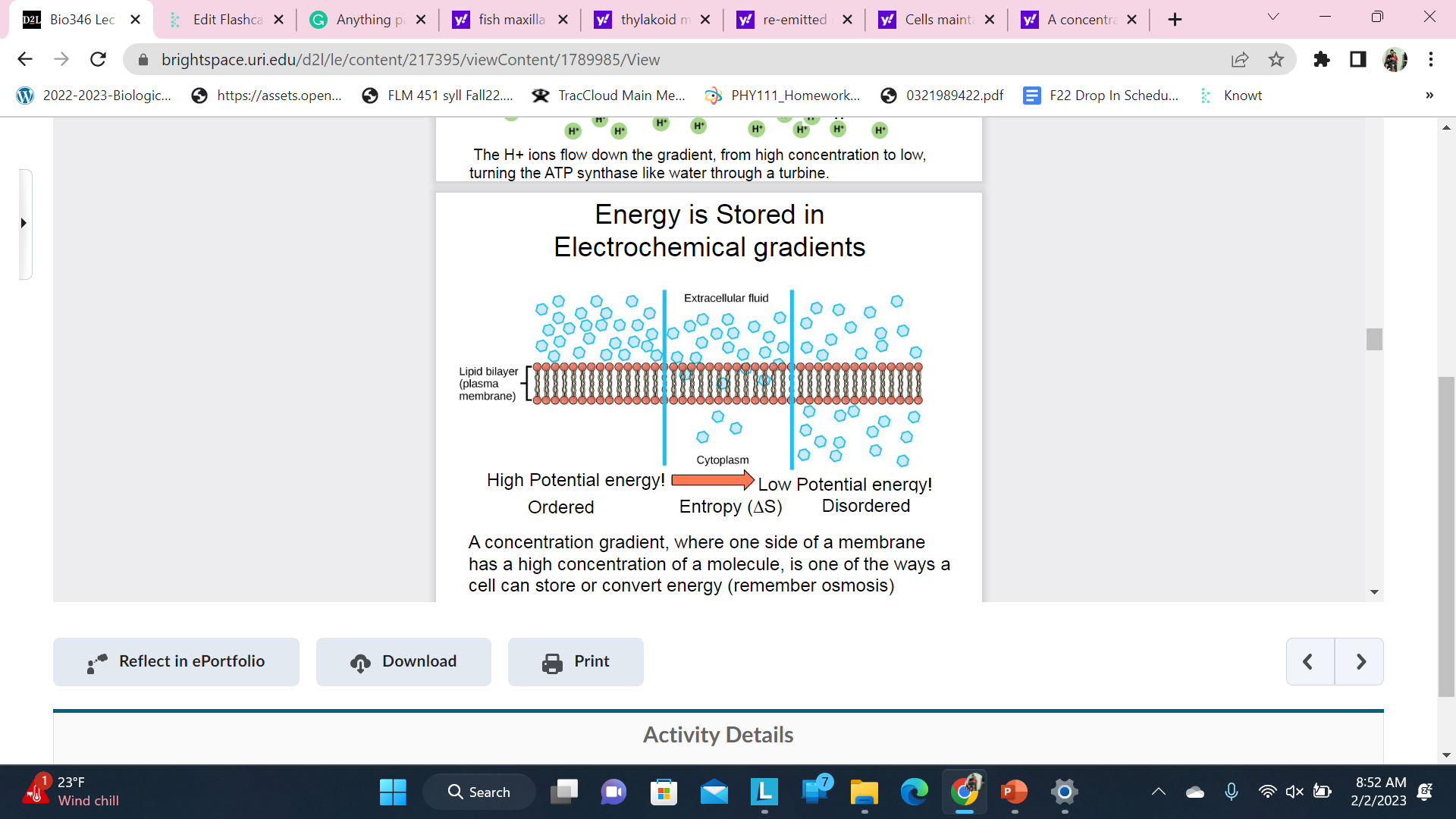
Energy is Stored in Electrochemical gradients
A concentration gradient, where one side of a membrane has a high concentration of a molecule, is one of the ways a cell can store or convert energy (remember osmosis)
40
New cards
Biological Membranes
A concentration gradient, where one side of a membrane has a high concentration of a molecule, is one of the ways a cell can store (hold) energy High Potential energy!
41
New cards
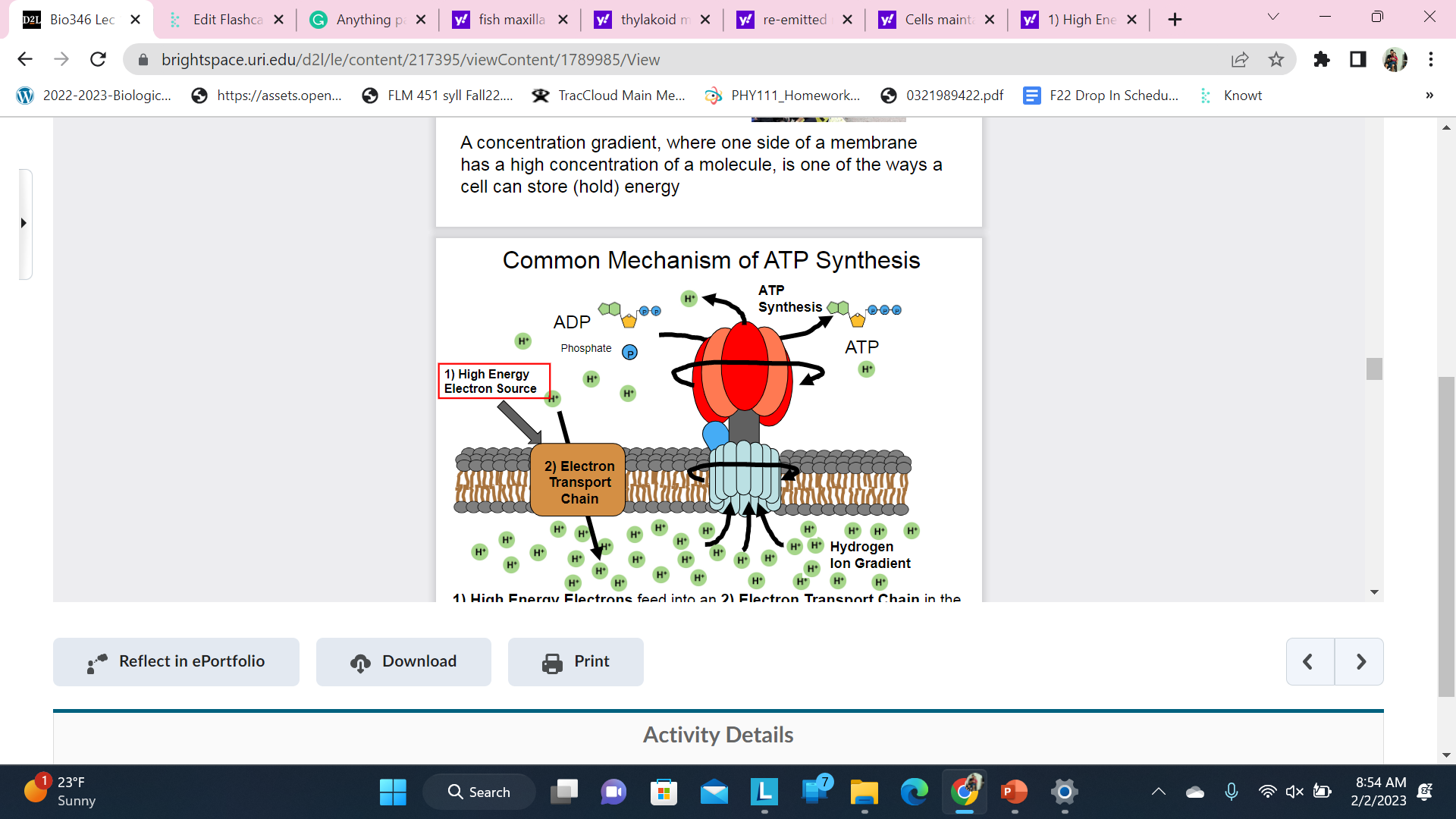
Common Mechanism of ATP Synthesis
1. High Energy Electrons feed into an 2) Electron Transport Chain in the membrane which uses the power to create a 3) Hydrogen Ion gradient that flows back across the membrane to power 4) ATP synthesis
42
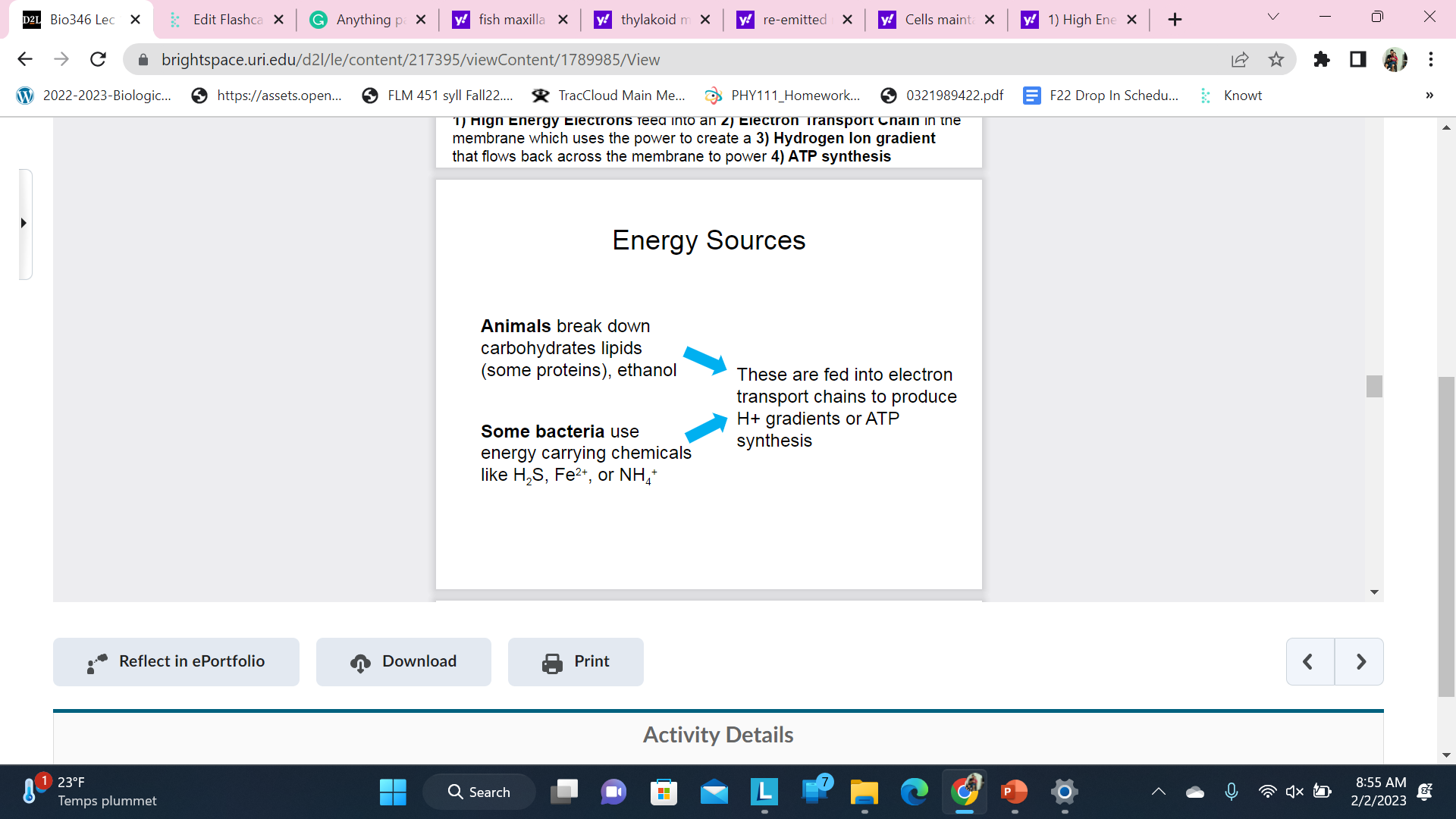
New cards
Energy Sources
43
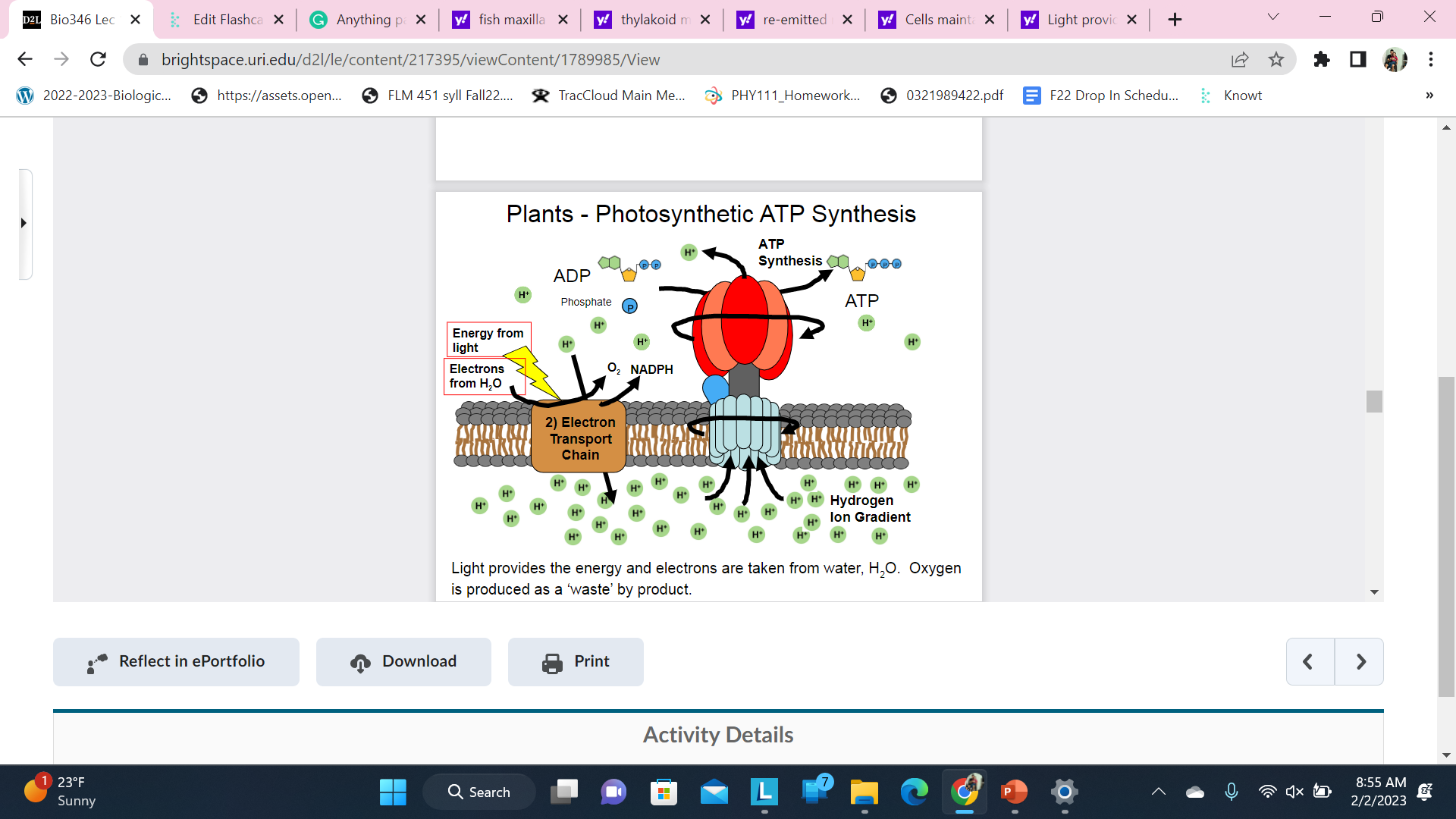
New cards
Plants - Photosynthetic ATP Synthesis
Light provides the energy and electrons are taken from water, H2O. Oxygen is produced as a ‘waste’ by product
44
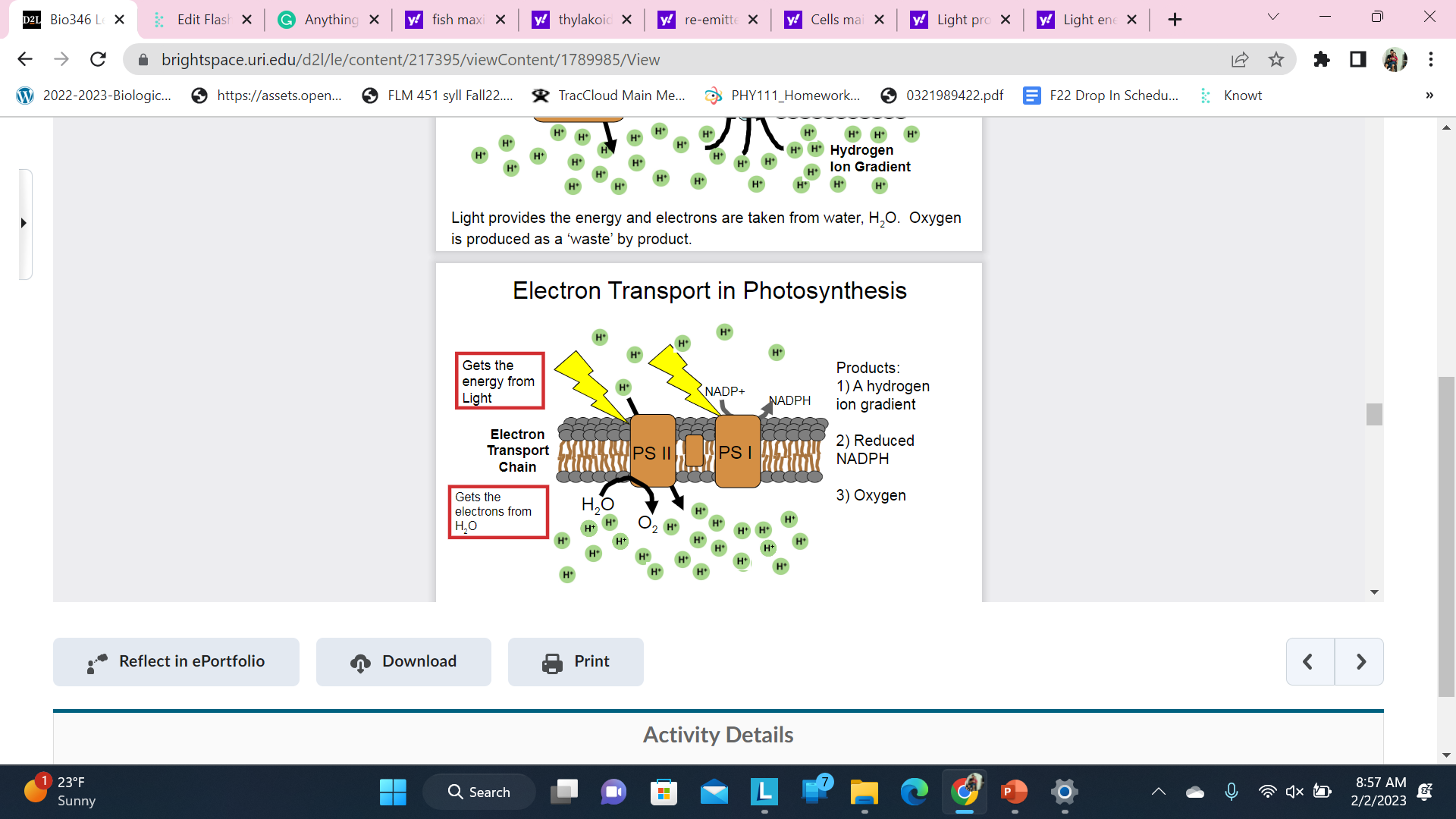
New cards
Electron Transport in Photosynthesis
Light energy enters the Electron Transport Chain in two places. Each energy entry point is called a Photosystem (or reaction center)
\
Know what carries energy and know what is done in each step and where it gets the electrons from
\
Know what carries energy and know what is done in each step and where it gets the electrons from
45
New cards
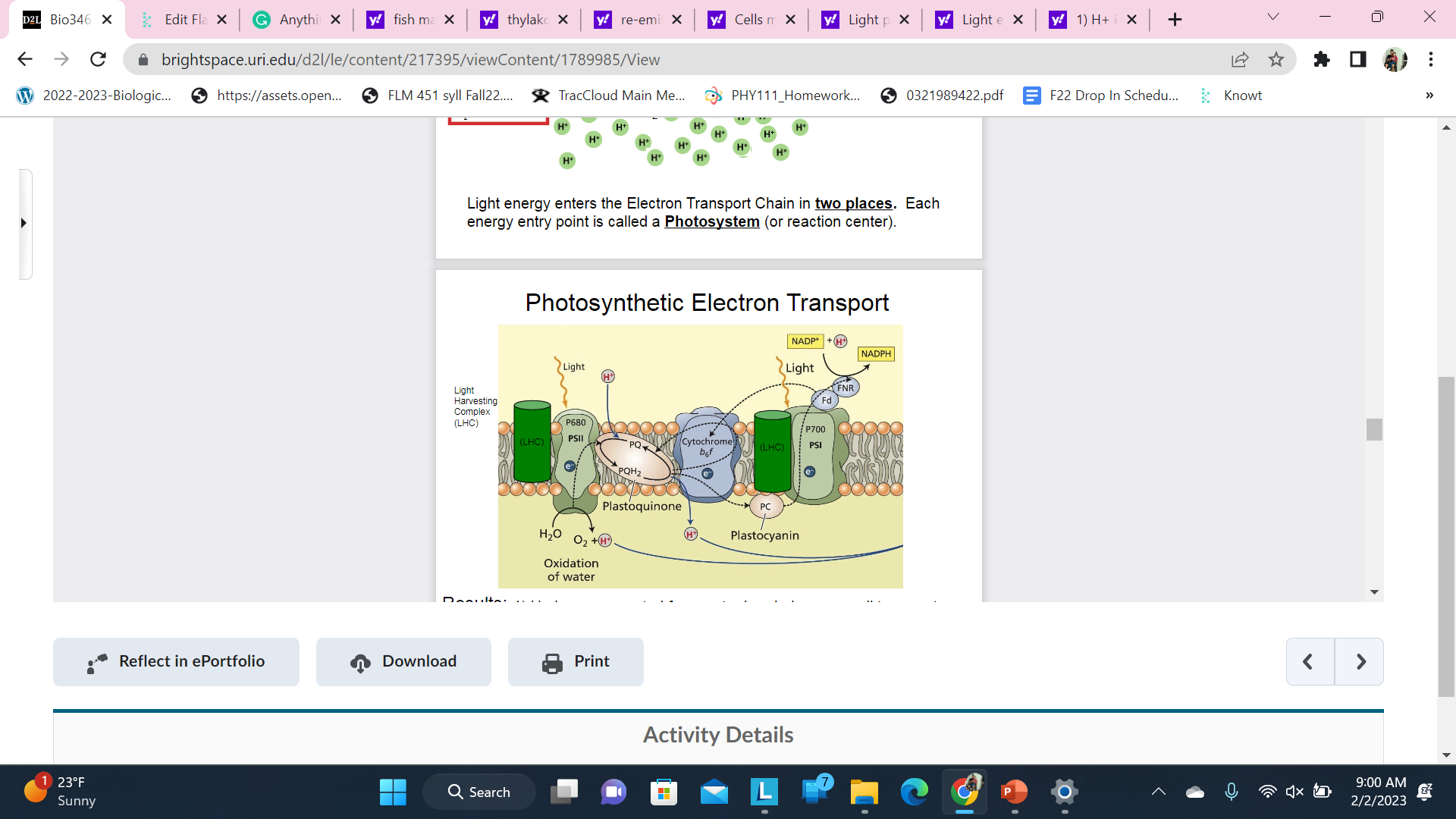
Photosynthetic Electron Transport;Thylakoids reaction
Results:
1. H+ ions are created from water break down as well transport across the membrane into the thylakoid lumen. (creates a gradient that can do work!)
2. 2) Creates reduced NADPH (high energy)
1. H+ ions are created from water break down as well transport across the membrane into the thylakoid lumen. (creates a gradient that can do work!)
2. 2) Creates reduced NADPH (high energy)
46
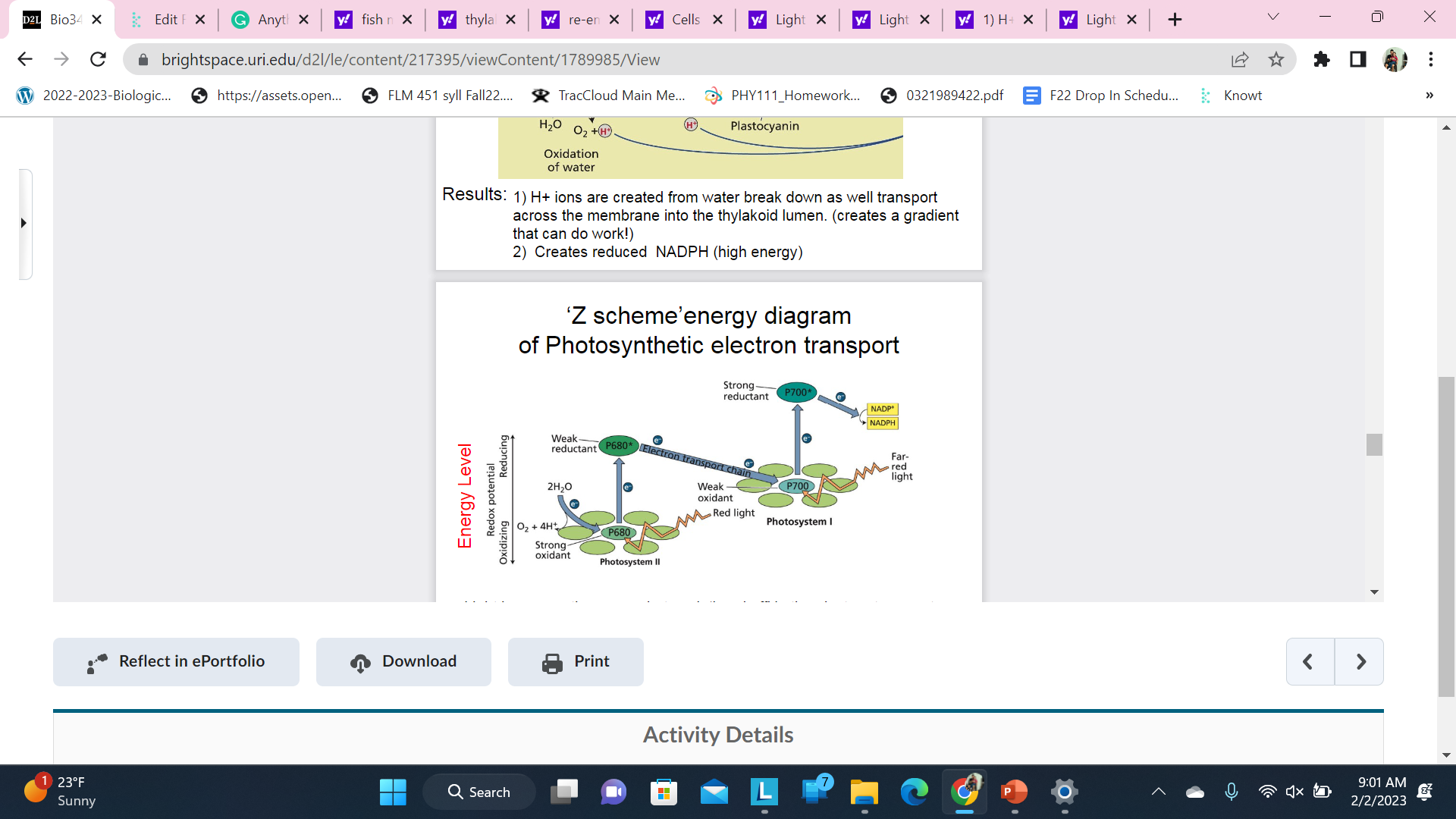
New cards
\n Z scheme’energy diagram \n of Photosynthetic electron transport
Light increases the energy but each ‘hand off’ in the electron transportchain looses some energy
\
After the second light entry the carriers are energized enough toreduce NADP+ to NADPH directl
\
After the second light entry the carriers are energized enough toreduce NADP+ to NADPH directl
47
New cards
Thylakoids reaction;Products of electron transport
1) Oxidize H2O to O2
2) Reduction of NADP+ to NADPH
3) Produce of NADP+ to NADPH
\
H+ from 1) oxidizing H2O to O2 \n
\
H+ transported to thylakoid Lumen by plastoquinone Q \n
H+ taken up by NADPH formation \n
2) Reduction of NADP+ to NADPH
3) Produce of NADP+ to NADPH
\
H+ from 1) oxidizing H2O to O2 \n
\
H+ transported to thylakoid Lumen by plastoquinone Q \n
H+ taken up by NADPH formation \n
48
New cards
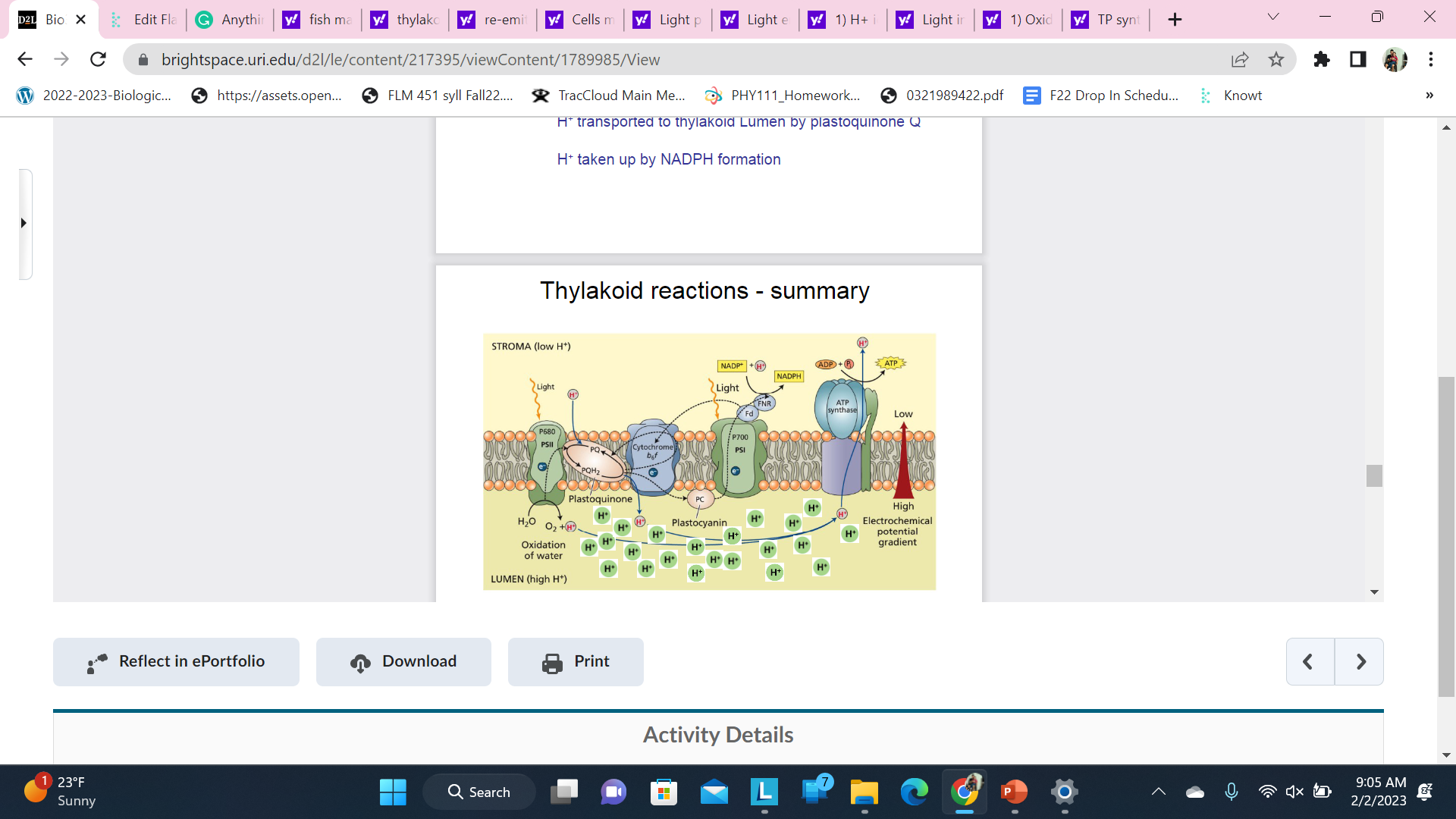
Thylakoid reactions - summary
ATP synthesis is not part of electron transport. ATP synthesis is coupled to electron transport by the H+ gradient
49
New cards
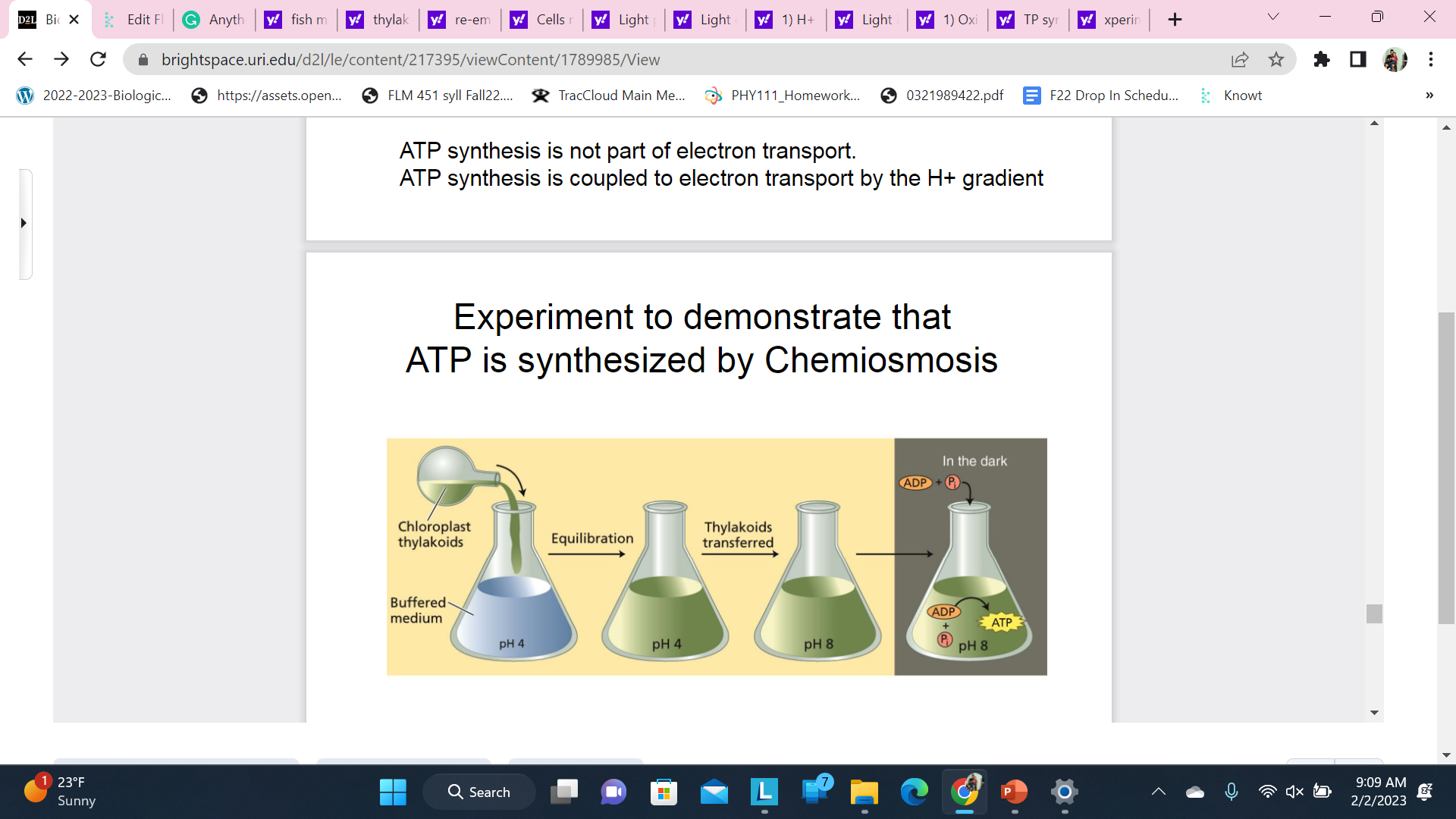
Experiment to demonstrate that ATP is synthesized by Chemiosmosis
Scientists created an artificial gradient of H+ ions across the thylakoid membrane and were able to power ATP synthesis (light or dark)
50
New cards
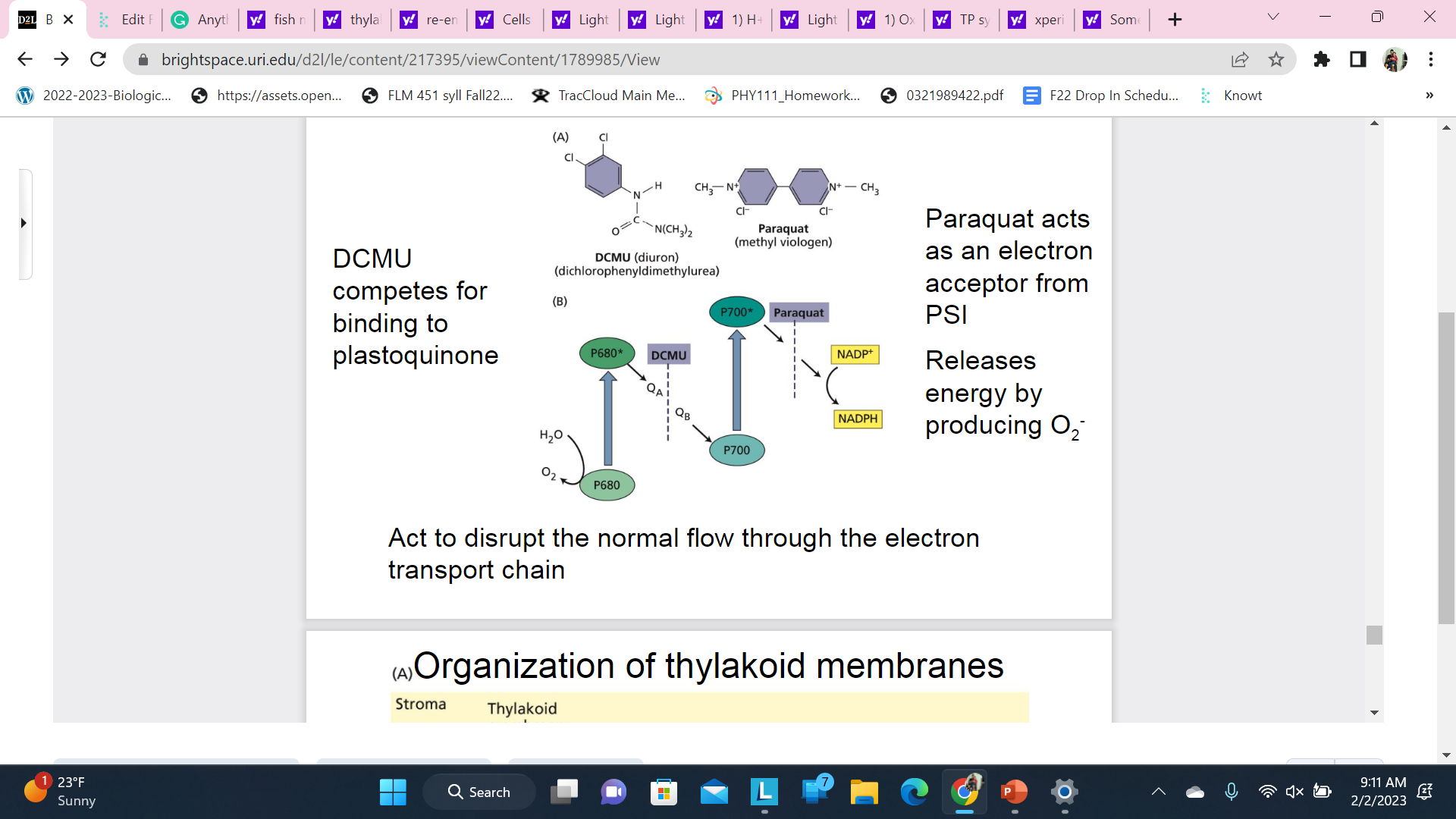
Some Herbicides act to kill plants by blocking photosynthetic electron transport
DMCU blocks the transition
\
Produces free radicals
\
Produces free radicals
51
New cards
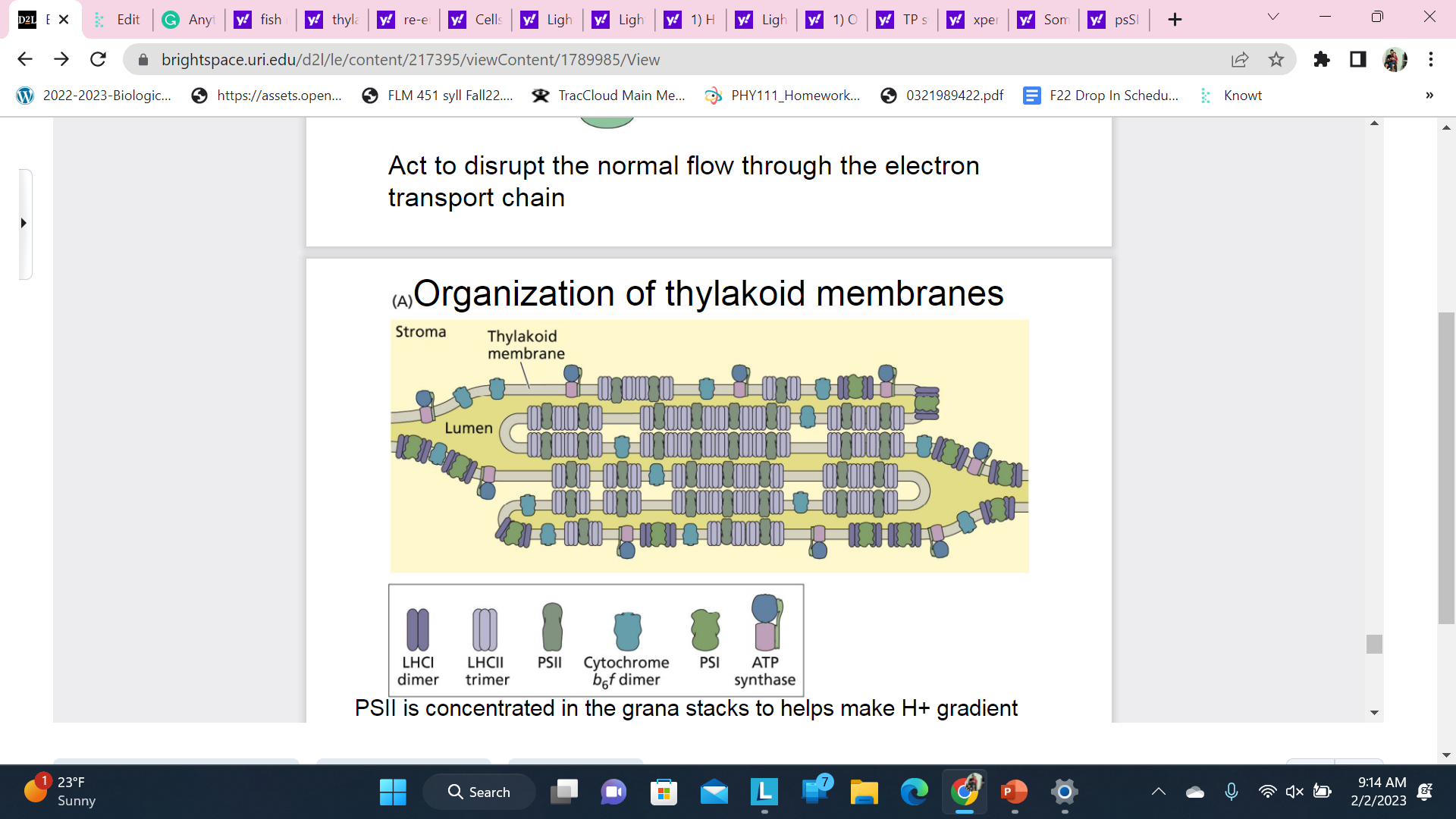
Organization of thylakoid membranes
PSSII is concentrated in the grana stacks to helps make H+ gradient - less volume to fill means concentration increases with fewer H+ ions
\
PSI and ATPase are concentrated in the stromal lamellae so products can diffuse away to be used
\
Cytochrome and other carriers transport e- between photosystems
\
PSI and ATPase are concentrated in the stromal lamellae so products can diffuse away to be used
\
Cytochrome and other carriers transport e- between photosystems