Chem Chapters 13, 14 Williams
5.0(1)
5.0(1)
Card Sorting
1/43
Earn XP
Description and Tags
States of Matter and the Behavior of Gasses (Gas Laws)
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
44 Terms
1
New cards
kinetic energy
energy of motion
2
New cards
KE formula
* KE = ½ mv^2 = 3/2 kT
* k = boltzmann’s constant (maxwell - boltzmann’s distribution)
* T = temperature in kelvin
* m = mass (kg)
* v = velocity (m/s)
* v 𝜶 T
* k = boltzmann’s constant (maxwell - boltzmann’s distribution)
* T = temperature in kelvin
* m = mass (kg)
* v = velocity (m/s)
* v 𝜶 T
3
New cards
“proportional to”
𝜶
4
New cards
sublimation
solid to gas
5
New cards
deposition
gas to solid
6
New cards
melting
solid to liquid
7
New cards
freezing
liquid to solid
8
New cards
evaporation
liquid to gas
9
New cards
condensation
gas to liquid
10
New cards
vaporization
solid or liquid to gas when boiling
11
New cards
gas assumptions
* Particles are small, hard spheres with insignificant volume
* Particles move in a straight line and random motion
* Particles colliding with other particles or the container walls are elastic
* In elastic collisions, particles bounce off each other like billiard balls
* When inelastic collisions take place, particles can stick together (bond) or deform from the impact (cars)
* Particles move in a straight line and random motion
* Particles colliding with other particles or the container walls are elastic
* In elastic collisions, particles bounce off each other like billiard balls
* When inelastic collisions take place, particles can stick together (bond) or deform from the impact (cars)
12
New cards
gas pressure
The result of gas particles striking the surface of the container
13
New cards
what is pressure measured in?
* Pressure (**kPa**) = force/area = newtons/m2
* Measurement unit for pressure: SI unit for pressure: Pascal (Pa) and NY State also uses atmospheres (atm)
* Units for pressure: in Hg, mm Hg, PSI, torr, bar
* 1 mmHg = 1 torr
* 30 in Hg = 760 mmHg = 760 torr = 14.7 psi = 1 atm
* Measurement unit for pressure: SI unit for pressure: Pascal (Pa) and NY State also uses atmospheres (atm)
* Units for pressure: in Hg, mm Hg, PSI, torr, bar
* 1 mmHg = 1 torr
* 30 in Hg = 760 mmHg = 760 torr = 14.7 psi = 1 atm
14
New cards
devices used to measure gas pressure
barometer, manometer, gauge
15
New cards
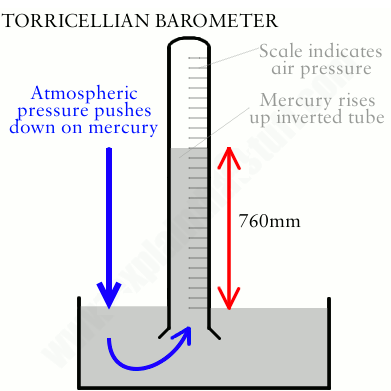
barometer
Pressure of atmosphere pushes down on mercury + based on the air pressure the mercury will rise or fall certain levels
16
New cards
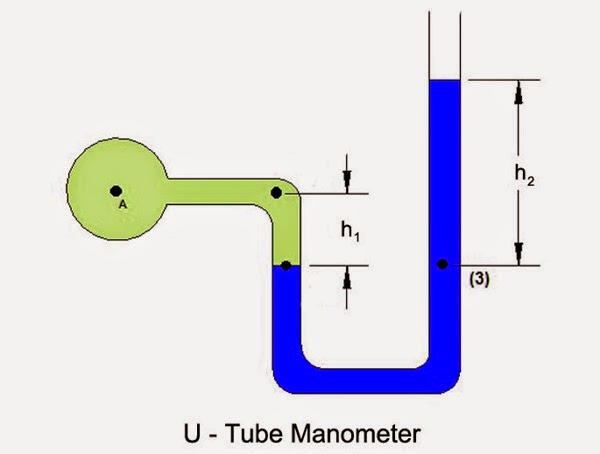
manometer
Fill with tested gas + high density liquid (aka mercury) to determine pressure - if pressure were the same, the blue levels would be parallel to each other
17
New cards
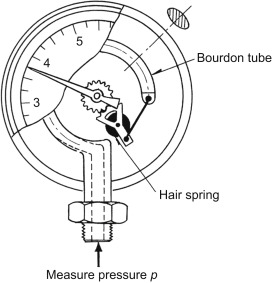
gauge
Pressure gauges work through a Bourdon tube, a hollow piece of metal. When a gas or liquid enters the tube, it expands and pushes a lever. The distance the lever moves is proportional to the gas or liquid pressure inside the hollow tube.
18
New cards
nature of liquids
*Intermolecular forces dictate the properties of liquids*
* Van der Waal forces
* Hydrogen bonding
* London Dispersion forces (LDF)
* Dipole - dipole
* Dipole - induced dipole
* Induced dipole - induced dipole
* Van der Waal forces
* Hydrogen bonding
* London Dispersion forces (LDF)
* Dipole - dipole
* Dipole - induced dipole
* Induced dipole - induced dipole
19
New cards
dipole
the electrons spending more time at one end of the molecule because of the charges attracting
20
New cards
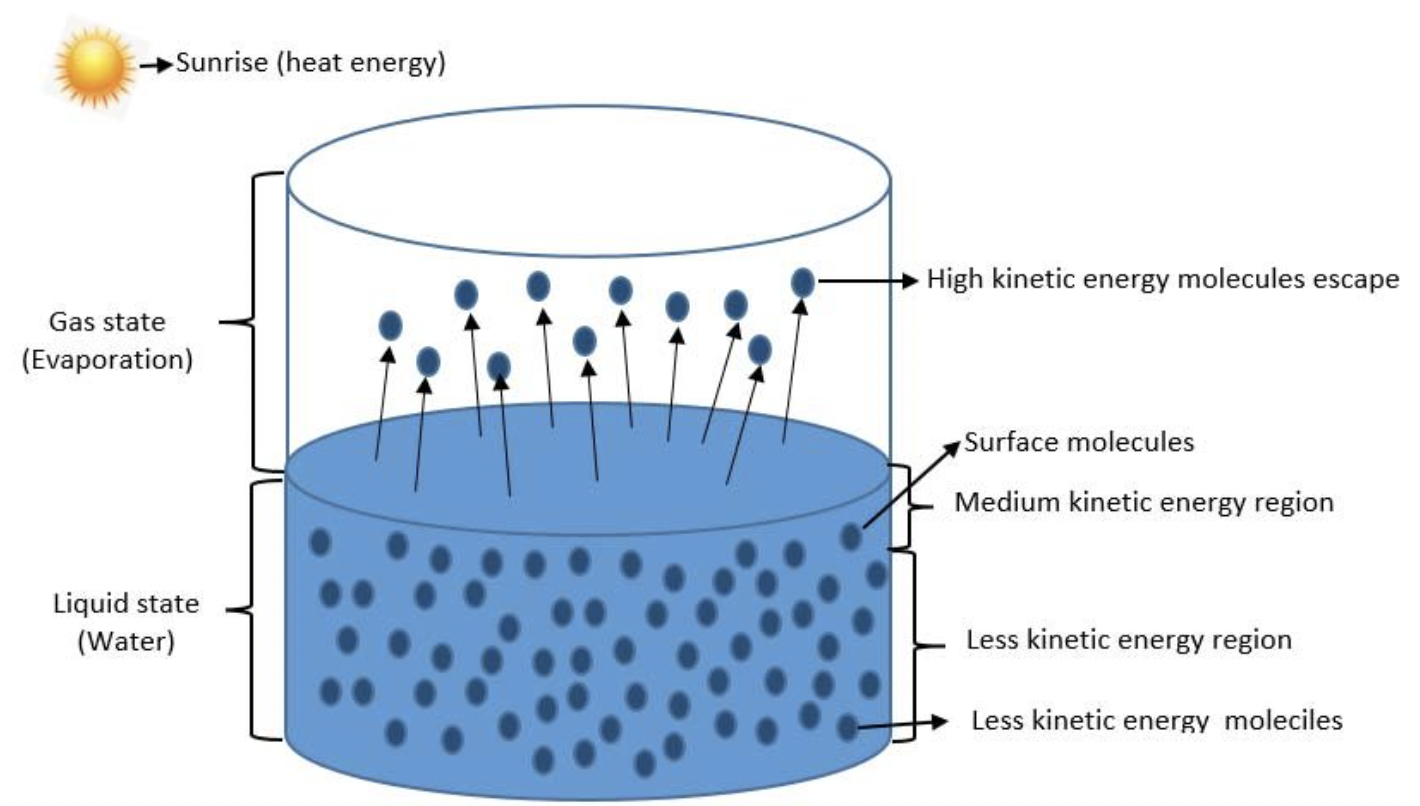
evaporation
* *Particles at the surface of a liquid have sufficient kinetic energy to “escape” the intermolecular forces*
* Liquid temperature is well below the boiling temperature
* Temperature of liquid particles follow a “normal distribution”
* Particles on the right have enough energy (temperature) to overcome the intermolecular forces holding them in the liquid
* Liquid temperature is well below the boiling temperature
* Temperature of liquid particles follow a “normal distribution”
* Particles on the right have enough energy (temperature) to overcome the intermolecular forces holding them in the liquid
21
New cards
boiling point
**temperature when the atmospheric pressure is equal to the vapor pressure**
* At the boiling point, there is gas inside the liquid (bubbles)
* The boiling point of a liquid is not a specific value
* The boiling point changes based upon the atmospheric pressure
* The “Normal Boiling Point” is the temperature that a liquid boils when the pressure is 101.3 kPa or 1.00 atm
* At the boiling point, there is gas inside the liquid (bubbles)
* The boiling point of a liquid is not a specific value
* The boiling point changes based upon the atmospheric pressure
* The “Normal Boiling Point” is the temperature that a liquid boils when the pressure is 101.3 kPa or 1.00 atm
22
New cards
Vapor pressure
* When particles “escape” the liquid, they become gas particles
* These gas particles generate a pressure
* *As temperature increases, the number of particles escaping the liquid increases*
* *More particles mean that they will strike the surface of the pressure sensor*
* *Measurement of pressure increases*
* *Manometer commonly used to measure vapor pressure*
* In an open container, the gas particles can drift away which allows more liquid particles to evaporate
* In a closed container, the gas particles are trapped. If the gas particles lose enough energy through collisions, they can be “recaptured” by the liquid
Establishes a **dynamic equilibrium**
* These gas particles generate a pressure
* *As temperature increases, the number of particles escaping the liquid increases*
* *More particles mean that they will strike the surface of the pressure sensor*
* *Measurement of pressure increases*
* *Manometer commonly used to measure vapor pressure*
* In an open container, the gas particles can drift away which allows more liquid particles to evaporate
* In a closed container, the gas particles are trapped. If the gas particles lose enough energy through collisions, they can be “recaptured” by the liquid
Establishes a **dynamic equilibrium**
23
New cards
model for solids
Solids reflect an organized arrangement of the particles in fixed locations
* It is important to remember that the particles in the solid are in constant vibrational, rotational, and very limited translational motion
* The lower the temperature goes, the slower these movements become
* At 0 K, all movement of the particles theoretically “stops”.
* It is important to remember that the particles in the solid are in constant vibrational, rotational, and very limited translational motion
* The lower the temperature goes, the slower these movements become
* At 0 K, all movement of the particles theoretically “stops”.
24
New cards
How does 0 K violate the conservation of energy law?
law says energy can’t be created nor destroyed - 0K is saying that there is essentially no energy left which can never be true
25
New cards
unit cell
All solids are build on one of these structures
* Each unit cell has unique properties
* When exposed to heat, solids can change the unit cell
* Each unit cell has unique properties
* When exposed to heat, solids can change the unit cell
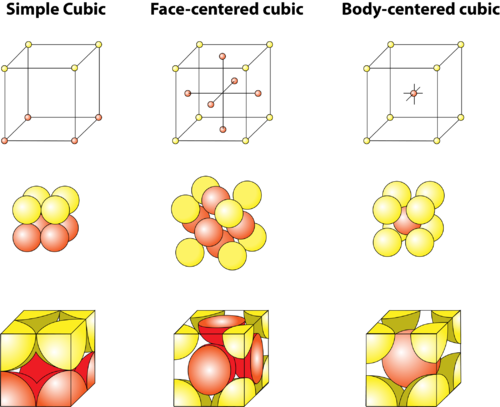
26
New cards
Simple Cubic
tends to be fragile
27
New cards
Face centered cubic (FCC)
tend to be strong but brittle
28
New cards
Body centered cubic (BCC)
tends to be strong and elastic
29
New cards
crystal systems
All solids are build around one of these seven crystal systems - cubic, tetragonal, monoclinic, orthorhombic, triclinic, hexagonal, trigonal
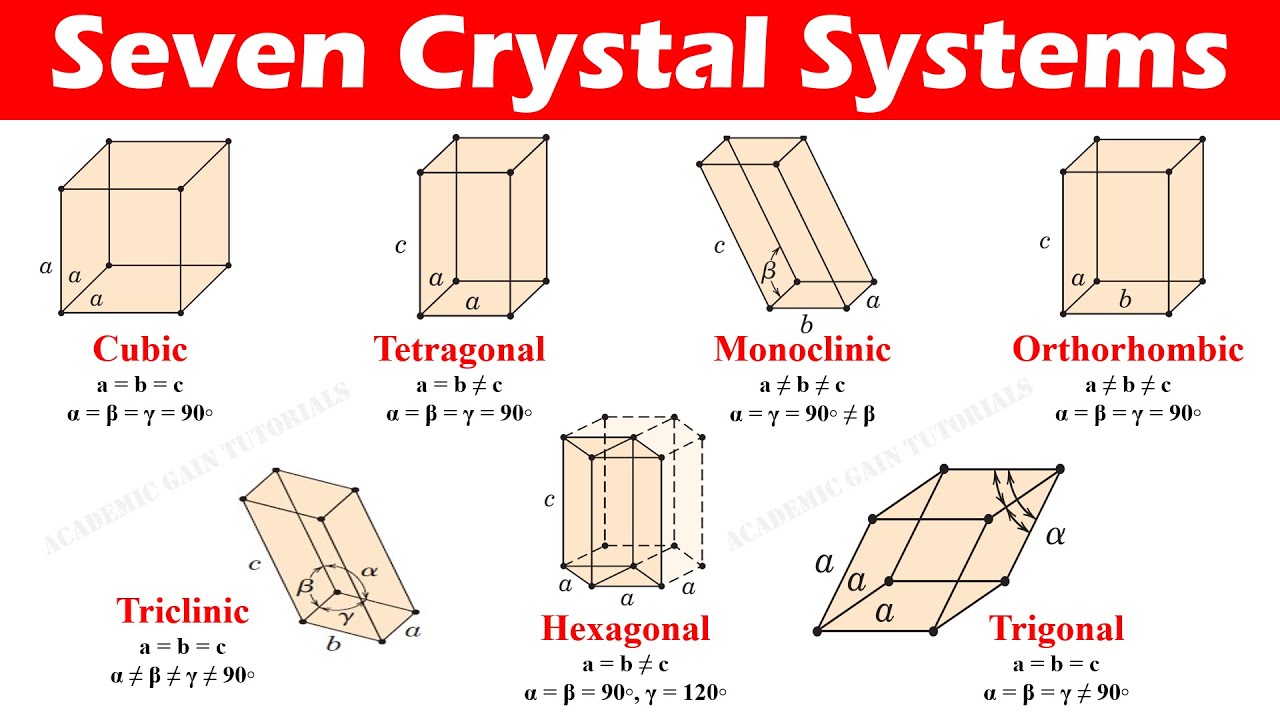
30
New cards
allotrope
substance that is made from the same elements but can form two or more different structures
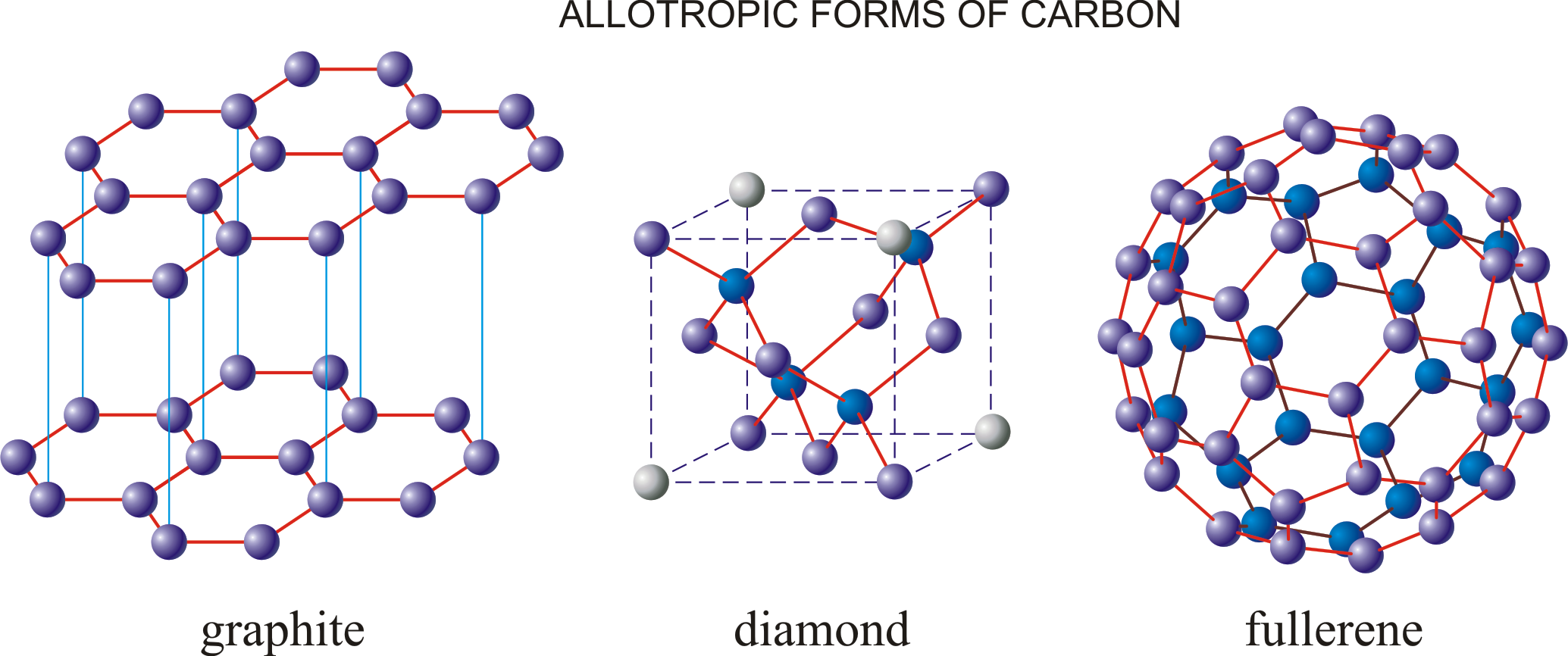
31
New cards
amorphous solid
a substance that lacks an ordered internal structure
* Glass is an example of an amorphous solid
* Glass is an example of an amorphous solid
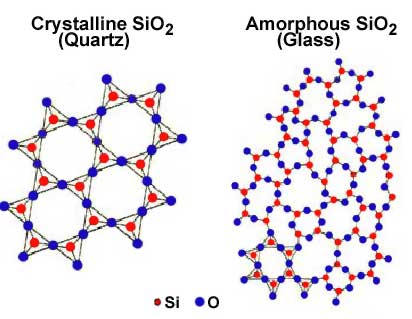
32
New cards
Phase Diagrams
Shows the relationship between phase changes as a function of temperature and pressure
* Every substance has a phase diagram describing:
* **melting/freezing point**
* **boiling /condensation point**
* **Triple point: point where all three phases coexist**
**Sublimation range:** range when change from gas to solid
* Every substance has a phase diagram describing:
* **melting/freezing point**
* **boiling /condensation point**
* **Triple point: point where all three phases coexist**
**Sublimation range:** range when change from gas to solid
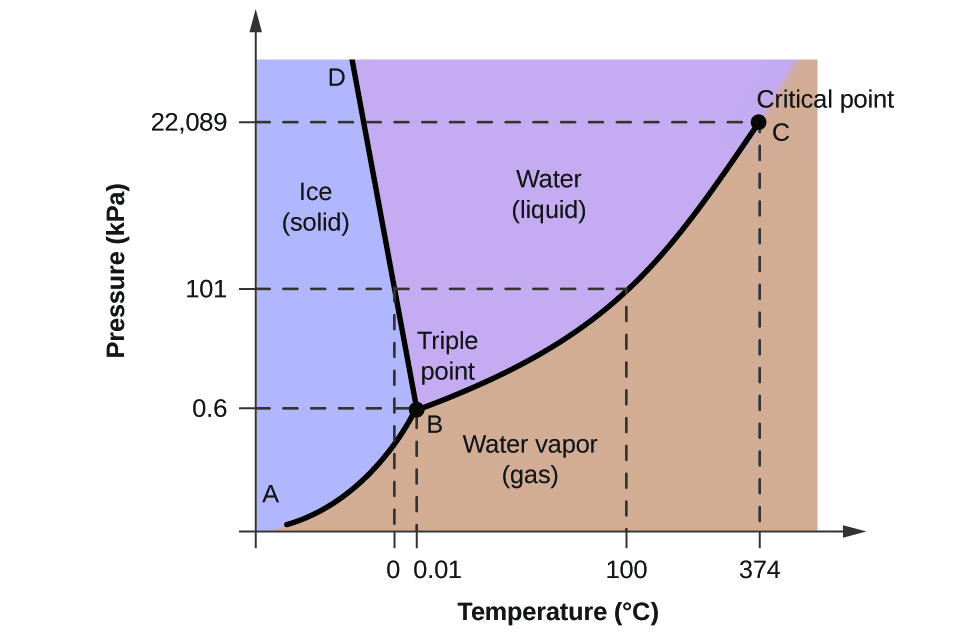
33
New cards
critical point
where substance enters plasma phase
34
New cards
property of gas: compressibility
Since gas particles are “far” apart, they can be forced closer together without dramatically altering the properties of the gas
* Pressure and density increase when a gas is compressed, but it remains a gas
* Pressure and density increase when a gas is compressed, but it remains a gas
35
New cards
What factors affect gas pressure?
Kinetic Theory of Gasses can be used to understand these factors
* **Amount** of gas
* The quantity of gas inside the container
* **Volume** of the container
* The volume of the container
* **Temperature** of the gas
* Temperature is a measurement of the average kinetic energy
* **Amount** of gas
* The quantity of gas inside the container
* **Volume** of the container
* The volume of the container
* **Temperature** of the gas
* Temperature is a measurement of the average kinetic energy
36
New cards
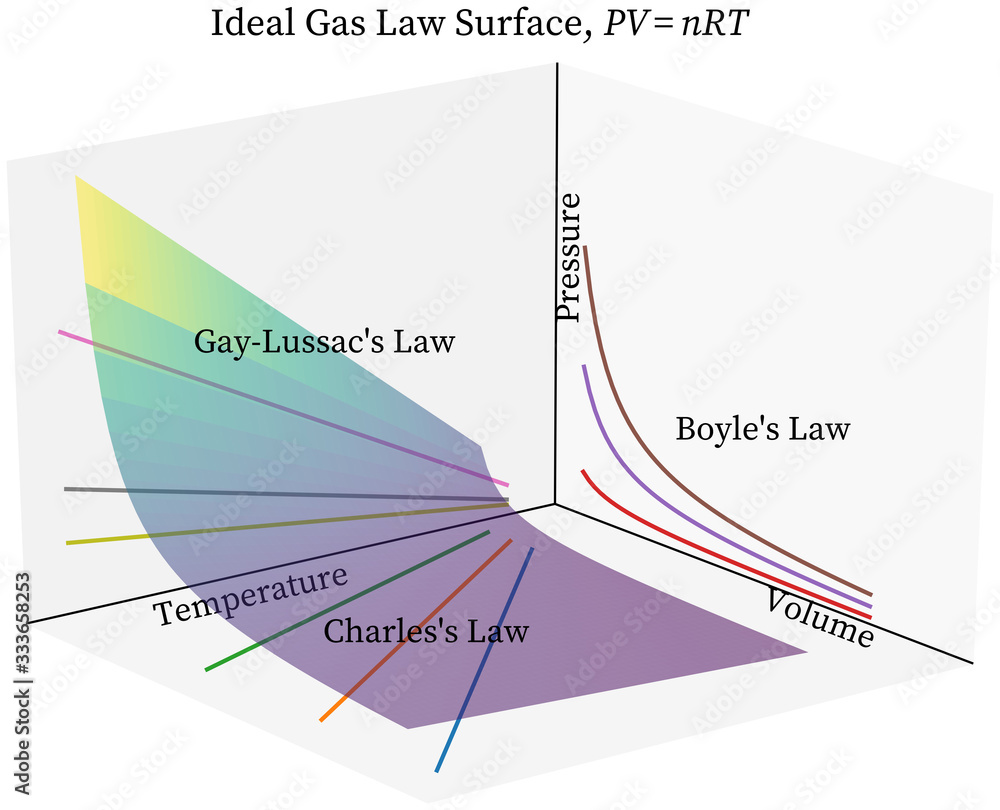
Boyle’s Law
as volume increases, pressure decreases
* Pressure (kPa/atm) vs Volume (L) - TEMPERATURE AND N ARE CONSTANT
* P1 x V1 = P2 x V2
* Indirect relationship
* Pressure (kPa/atm) vs Volume (L) - TEMPERATURE AND N ARE CONSTANT
* P1 x V1 = P2 x V2
* Indirect relationship
37
New cards
Charles’ Law
as temperature increases, volume increases - PRESSURE AND N ARE CONSTANT
* Volume (L) vs Temperature (K)
* V1/T1 = V2/T2
* Direct relationship
* *Must use Kelvin scale because does not have negative values*
* Volume (L) vs Temperature (K)
* V1/T1 = V2/T2
* Direct relationship
* *Must use Kelvin scale because does not have negative values*
38
New cards
Gay-Lussac’s Law
as temperature increases, pressure increases
* Pressure (kPa/atm) vs Temperature (K)
* P1/T1 = P2/T2
* Direct relationship
* *Must use Kelvin scale*
* Pressure (kPa/atm) vs Temperature (K)
* P1/T1 = P2/T2
* Direct relationship
* *Must use Kelvin scale*
39
New cards
Combined Gas Law
When all three responses are combined with a fixed amount of gas
* Moles of gas are constant
* Allows for each variable to be manipulated independently
* PV/T = K
* Moles of gas are constant
* Allows for each variable to be manipulated independently
* PV/T = K
40
New cards
The Ideal Gas Law
expands the Combined Gas Law to include the variable of matter - moles
* PV/T = constant
* PV/nT = R
* n is the mole value of the gas
* R is the universal gas constant
* **R = 8.31L•kPa/mol•K = 0.0821 L•atm/mol•K**
* PV/T = constant
* PV/nT = R
* n is the mole value of the gas
* R is the universal gas constant
* **R = 8.31L•kPa/mol•K = 0.0821 L•atm/mol•K**
41
New cards
Dalton’s Law
**The total pressure is the sum of the partial pressures of each gas in the mixture**
* PT = P1 + P2 + P3 + …
* PT = P1 + P2 + P3 + …
42
New cards
Graham’s Law
*Describes the rate one gas will move through another gas*
* RateA/RateB = √ (molar massB/molar massA)
* RateA/RateB = √ (molar massB/molar massA)
43
New cards
Effusion
particles having a barrier, when removed they randomly mix - or particles escaping through a hole in barrier (pinhole) due to differences in pressure inside and outside
44
New cards
Diffusion
gas particles move from higher concentration to lower concentration to reach equilibrium