Quantum
0.0(0)
0.0(0)
Card Sorting
1/9
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
10 Terms
1
New cards
energy per oscillator
U=kBT
2
New cards
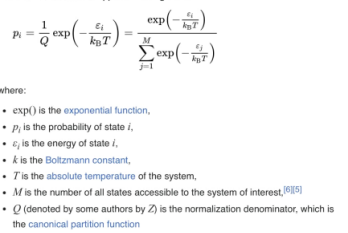
Boltzmann distribution
a probability distribution that gives the probability of a certain state as a function of that state’s energy and temp of the system to which distribution is applied
3
New cards
oscillation Nj
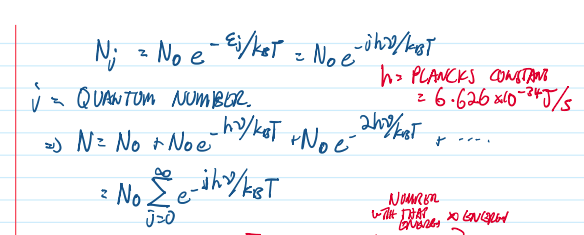
4
New cards
Total energy is then
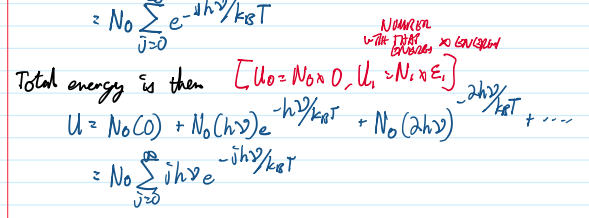
5
New cards
average energy is then
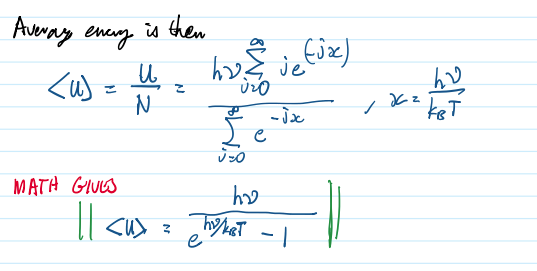
6
New cards
if atom is considered a crystal-3 directions of motion- 3 oscillators
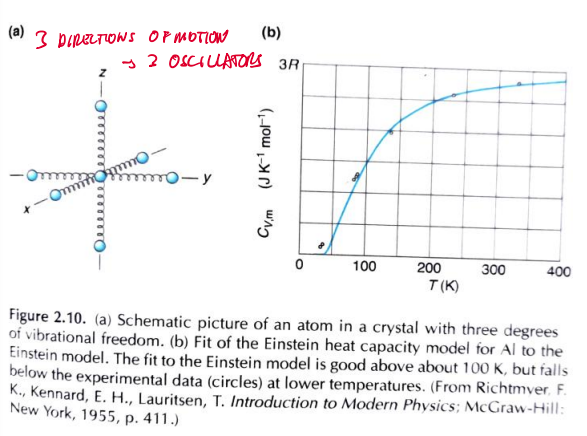
7
New cards
total energy per mole
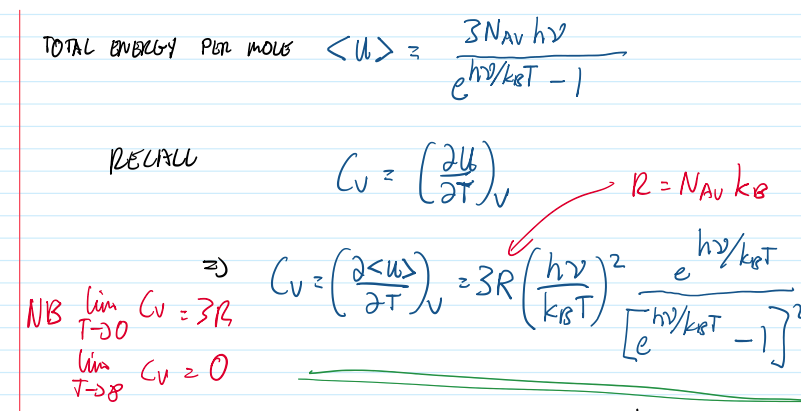
8
New cards
plancks distribution and einstein heat capacity frequency oscillator population
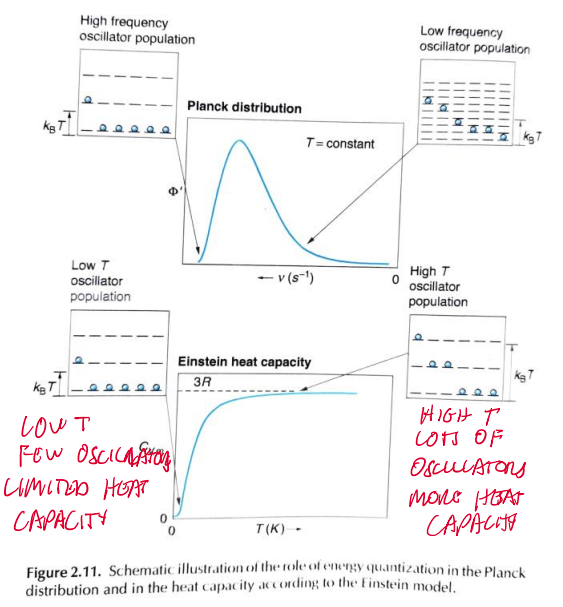
9
New cards
wave particle duality
electrons through a double slit at significant rates.
constructive interference giving maxima and destructive interference giving minima.
electrons behave like waves
single slit-electrons act like particles
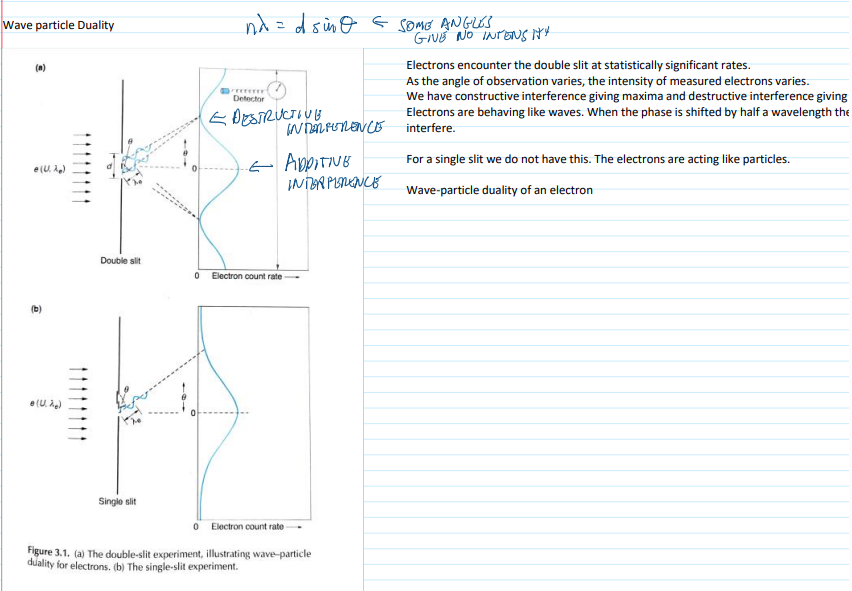
10
New cards