chapter 5.5 formation of spectral lines & 5.6 the doppler effect
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
8 Terms
electron orbitals
each type of atom has its own unique pattern of electron orbits, and no two sets of orbits are exactly alike
each type of atom shows its own unique set of spectral lines, produced by electrons moving between is unique set of orbits
ground state
an atom is in the state of lowest possible energy
in the Bohr model of the hydrogen atom, the ground state corresponds to the electron being in the innermost orbit
excitation
when an atom absorbs energy, to raise it to a higher energy level
the atom is said to be in an excited state
generally an atom only remains excited for only a very brief time
ionisation
occurs when an electron can be completely removed from an atom
occurs during an excited state
creates an ion
if enough energy is available, the atom can be completely ionised, losing all of its electrons
The rate at which such collisional ionisations occur depends on the speeds of the atoms and hence on the temperature of the gas—the hotter the gas, the more of its atoms will be ionised
depends on the density of the gas
doppler effect
an increase (or decrease) in the frequency of sound, light, or other waves as the source and observer move towards (or away from) each other
The effect causes the sudden change in pitch noticeable in a passing siren, as well as the red shift seen by astronomers
produced only by a motion toward or away from the observer, called radial velocity
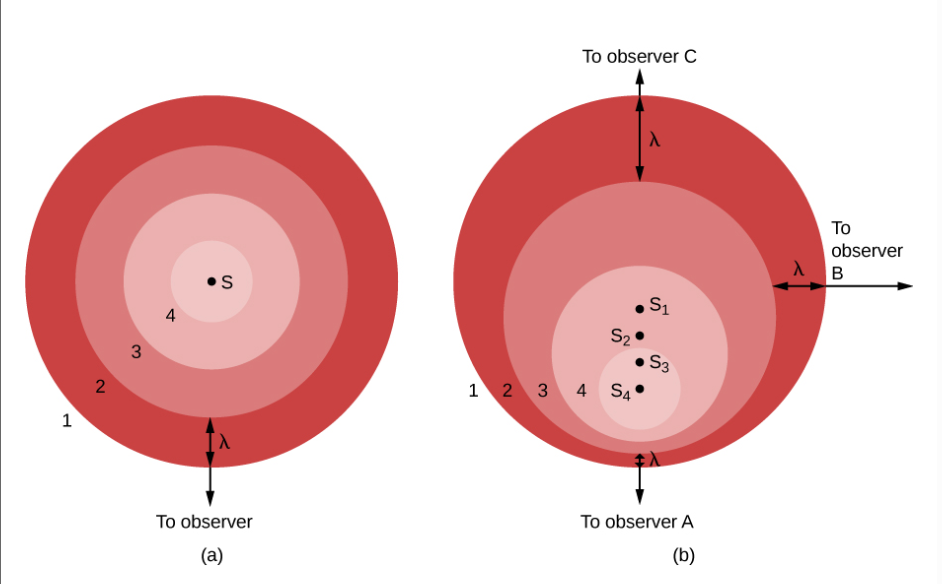
blueshift
When the source of waves moves toward you, the wavelength decreases a bit.
If the waves involved are visible light, then the colors of the light change slightly.
As wavelength decreases, they shift toward the blue end of the spectrum
redshift
When the source moves away from you and the wavelength gets longer
doppler effect formula