4.4 climate change
1/19
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
20 Terms
State the sources of CO2 and water vapor in the atmosphere.
Carbon dioxide is added to the atmosphere naturally when organisms respire or decompose, carbonate rocks are weathered, forest fires occur, and volcanoes erupt. Carbon dioxide is also added to the atmosphere through human activities, such as the burning of fossil fuels and forests and the production of cement.
The water in the atmosphere is due to evaporation of water during the water cycle. In the atmosphere, water exists as a gas (water vapor from evaporation), as a liquid (droplets of rain), and as a solid (snow and ice).
Outline the mechanism by which greenhouse gases trap heat in the atmosphere.
Solar energy absorbed at Earth’s surface is radiated back into the atmosphere as heat.
A layer of greenhouse gases in the atmosphere absorb the heat and radiate it back to the Earth's surface.
State two factors that determine the warming impact of a greenhouse gas.
1. Ability to absorb longwave radiation (only certain gases in the atmosphere have the ability to trap long wave radiation and therefore act as a greenhouse gas).
2. Abundance of the gas in the atmosphere (the amount of a particular gas in the atmosphere).
State two variables that determine the concentration of a gas in the atmosphere.
1. Rate of release of the gas into the atmosphere.
2. How long the gas persists in the atmosphere once it is there.
State how long water, methane and CO2 remain in the atmosphere, on average.
There are several processes that remove CO2 from the atmosphere. Most dissolves into the ocean over a period of 20–200 years. The rest is removed by slower processes that take up to hundreds of thousands of years, including chemical weathering and rock formation. This means that once in the atmosphere, carbon dioxide can continue to affect climate for thousands of years.
Methane is mostly removed from the atmosphere by chemical reaction, persisting for about 12 years.
Water vapour has a very short atmospheric lifetime, of the order of hours to days, because it is rapidly removed as rain and snow.
State that the Earth absorbs short-wave energy from the sun and re-emits longer wavelengths.
Solar energy enters Earth's atmosphere as shortwave radiation in the form of ultraviolet (UV) rays and visible light. Once in the Earth’s atmosphere, clouds and the surface absorb the solar energy. The ground heats up and re-emits energy as longwave radiation in the form of infrared rays.
Compare wavelengths of UV, visible and infrared radiation.
Electromagnetic energy travels as waves that vary in wavelength. Infrared radiation, what we experience as heat, has a longer wavelength than visible light. Ultraviolet has shorter wavelengths than visible light.
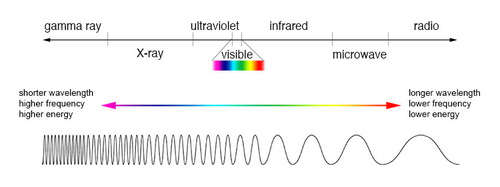
Explain the greenhouse effect, with reference to short wave radiation from the sun, long wave radiation from the Earth and the effects of ozone and greenhouse gases.
When the Sun’s shortwave energy reaches the Earth’s atmosphere, some of it is reflected back to space and the rest is absorbed and re-radiated as longwave radiation from the Earth's surface. Greenhouse gases in the atmosphere absorb some of the longwave radiation, which makes the atmosphere and Earth's surface warmer.
Explain why water vapour, CO2, methane and NO are greenhouse gases.
Water vapor, CO2, methane and N2O are greenhouse gases because they are able to absorb most of the Earth's emitted longwave infrared radiation, which heats the atmosphere.
Other atmospheric gases (such as N2 and O2) do not interact with longwave radiation, and therefore have no consequence for the greenhouse effect.
Explain why atmospheric greenhouse gas concentration would logically impact global temperatures.
The amount of greenhouse gases in the atmosphere is directly related to the temperature of the atmosphere. If the concentration of any of the greenhouse gases rises, more longwave radiation (heat) will be captured by the atmosphere leading to an increase in average global temperatures.
Outline the effect of global temperature on climate, specifically location and frequency of of rain and frequency of severe storms.
Higher average global temperatures are worsening many types of disasters, including storms, heat waves, floods, and droughts. A warmer climate creates an atmosphere that can collect, retain, and drop more water, changing weather patterns in such a way that wet areas become wetter and dry areas drier.
State the atmospheric CO2 concentration prior to the industrial revolution.
Before the Industrial Revolution, atmospheric levels of CO2 were around 280 parts per million. In 2013, the Mauna Loa observatory in Hawaii, which has been measuring atmospheric CO2 levels since 1958, recorded the milestone value of 400 parts per million of CO2 in the atmosphere, a level not seen since around 35 million years ago. The value continues to rise.
Outline the impact of the industrial revolution on atmospheric CO2 concentration.
The Industrial Revolution brought new industrial processes, an increase in the burning of fossil fuels, more extensive agriculture, and a rapid increase in the world's population. This rapid increase in human activity led to the emission of significant amounts of greenhouse gases into the atmosphere.
Describe the correlation between atmospheric CO2 concentrations since the industrial revolution and global temperatures.
There is a strong positive correlation between industrial processes (and the burning of fossil fuels) and the rising atmospheric concentrations of CO2.
In turn, the increase in atmospheric CO2 concentration correlates with an increase in average global temperature.
While correlation doesn't equal causation, there is substantial and growing evidence to suggest that CO2 emissions are linked to global average temperature increases.
Explain why industrial revolution would increase atmospheric CO2 concentrations.
Explain how historical temperature data has been collected.
Direct measurements of atmospheric gases have been made over the past 50 years. For historical data, analysis of air bubbles trapped in ancient ice, show that levels of carbon dioxide, methane, nitrous oxide and halocarbons are increasing.
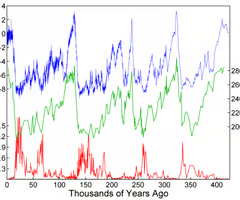
The Vostok ice core (from Antarctica) is one of the longest drilled, with ice dated to 420,000 years old. By analyzing the gas bubbles trapped in ice, historical CO2 levels and air temperatures can be deduced.
Using ice core data, outline the correlation between atmospheric CO2 concentration and global temperatures.
Scientists can study Earth’s climate as far back as 800,000 years by drilling core samples from deep underneath the ice sheets of Greenland and Antarctica. Detailed information on air temperature and CO2 levels is trapped in these specimens. Current polar records show a direct relationship between atmospheric carbon dioxide and global temperature.
Outline three reasons why there is vigorous debate around the claim that human activities are causing climate change.
The causes and effects of climate change have stirred vigorous debate because:
1. There is a degree of uncertainty in mathematical models used to predict consequences.
2. Climate patterns are complex with many variables.
3. Possible solutions to climate change will cost money and require government regulation.
4. Economic dependence on a fossil fuel based economy.
Outline the effect of atmospheric CO2 concentration on ocean pH.
Atmospheric CO2 dissolves into ocean water. The CO2 reacts with water molecules (H2O) to form carbonic acid (H2CO3). This compound then breaks down into a hydrogen ion (H+) and bicarbonate (HCO3-). The presence of all the hydrogen ions is what decreases the pH, or acidifies the ocean.
Describe the impact of lower ocean pH on animals that make skeletons from calcium carbonate.
Many marine organisms (such as coral, oysters, clams and snails) combine calcium and carbonate to form hard shells and skeletons out of the mineral calcium carbonate, CaCO3. Increased acidity slows the growth of calcium carbonate structures, and under severe conditions, can dissolve structures faster than they form.