Chapter 16: Electron Transport and Oxidative Phosphorylation, Biochemistry
1/48
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
49 Terms
Overview: Oxidative Fuel Metabolism
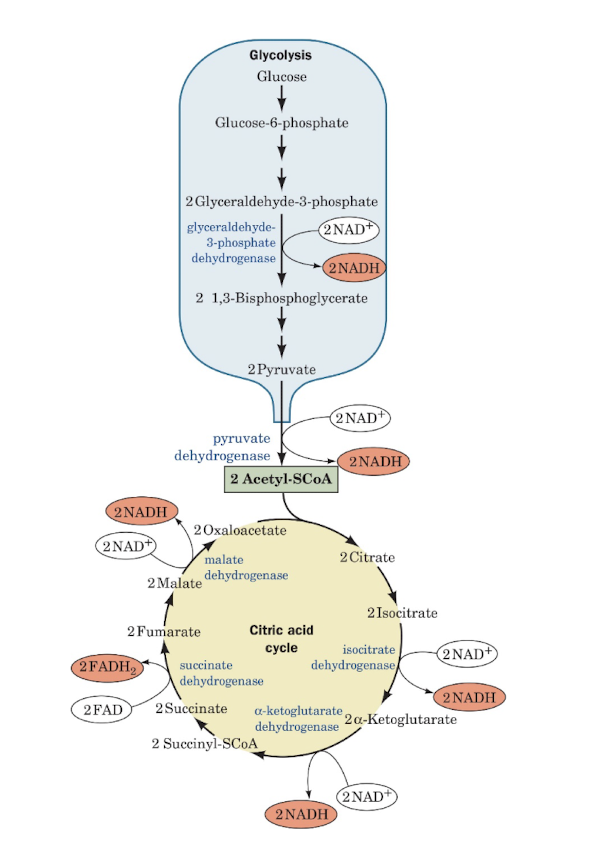
Learning Outcomes, 16.1: The Mitochondrion
explain how the structure and components of the mitochondrion contribute to its function.
Describe the internal structures of mitochondria.
Explain that transport proteins are required to import ADP and Pi into the mitochondria.
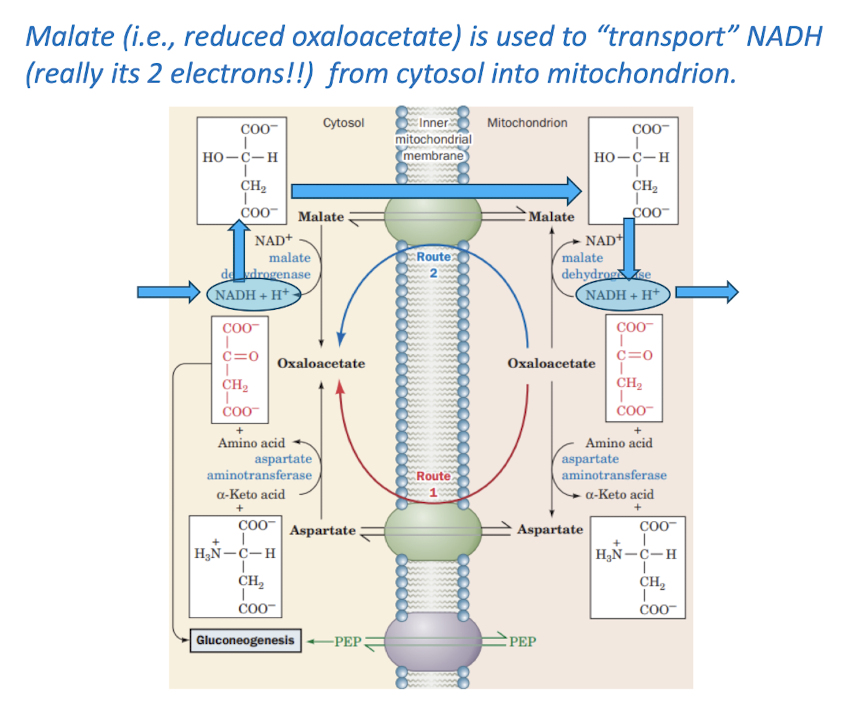
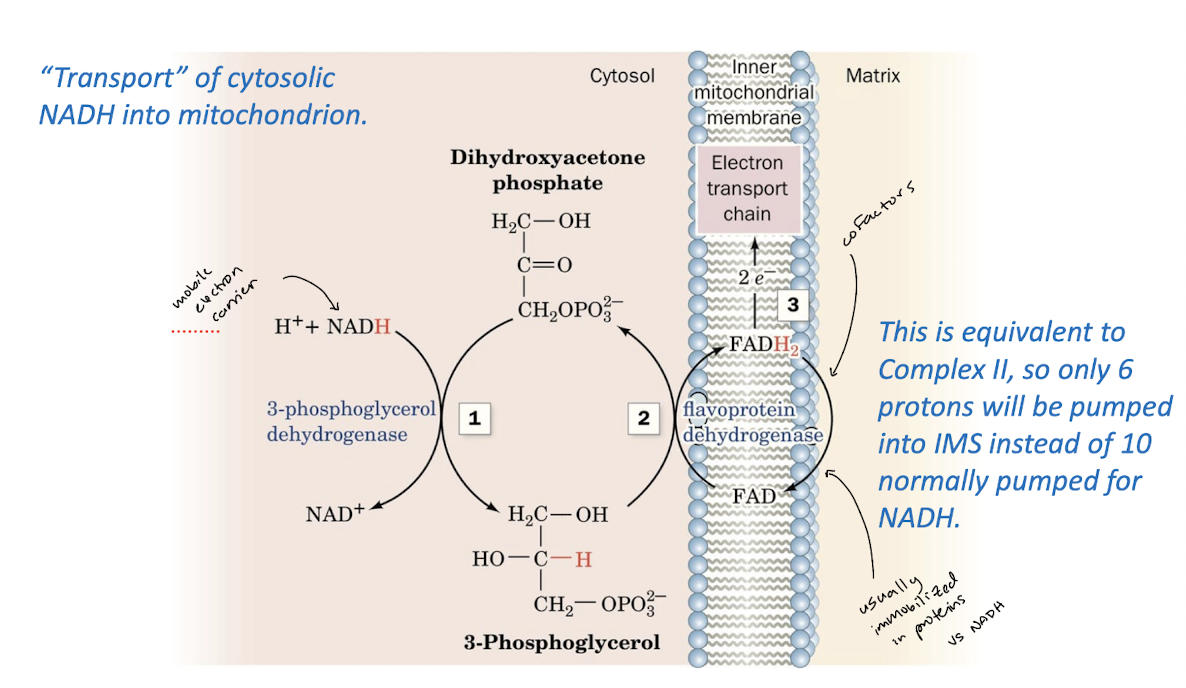
Identify how shuttle systems transport the electrons carried by NADH across the mitochondrial membrane.
Key Concepts, 16.1: The mitochondrion
Electrons from the reduced coenzymes NADH and FADH2 pass through a series of redox centers in the electron-transport chain before reducing O2.
During electron transfer, protons are translocated out of the mitochondrion. The free energy of the electrochemical gradient is converted into motion in ATP synthase that drives phosphorylation of ADP.
The mitochontrion contains soluble and membrane- bound enzymes for oxidative metabolism.
Reducing equivalents are imported from cytosol via a shuttle system. Specific transporters mediate the transmembrane movements of ADP, ATP, and Pi.
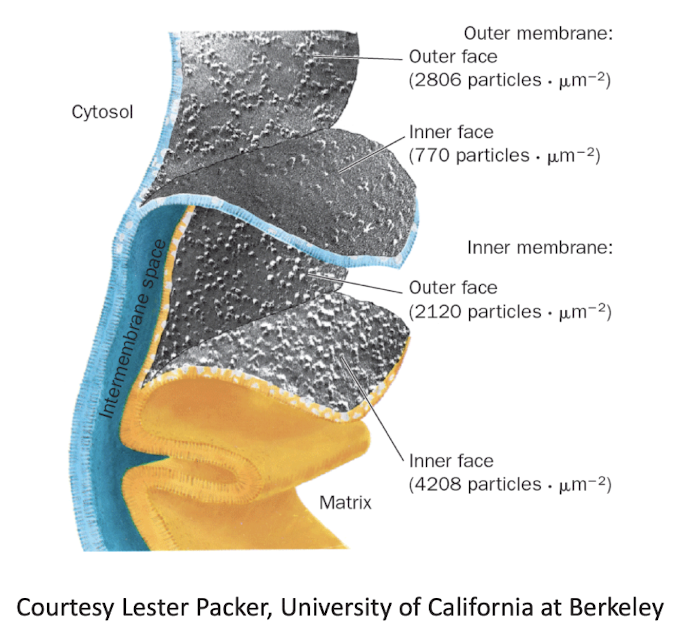
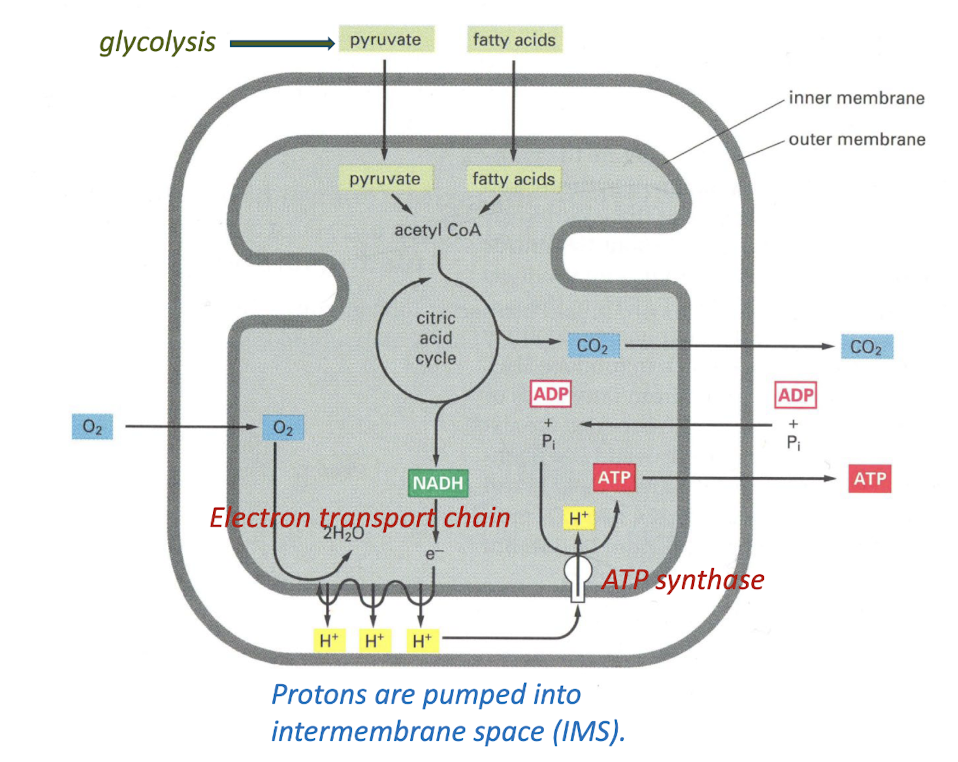
The Mitochondrion
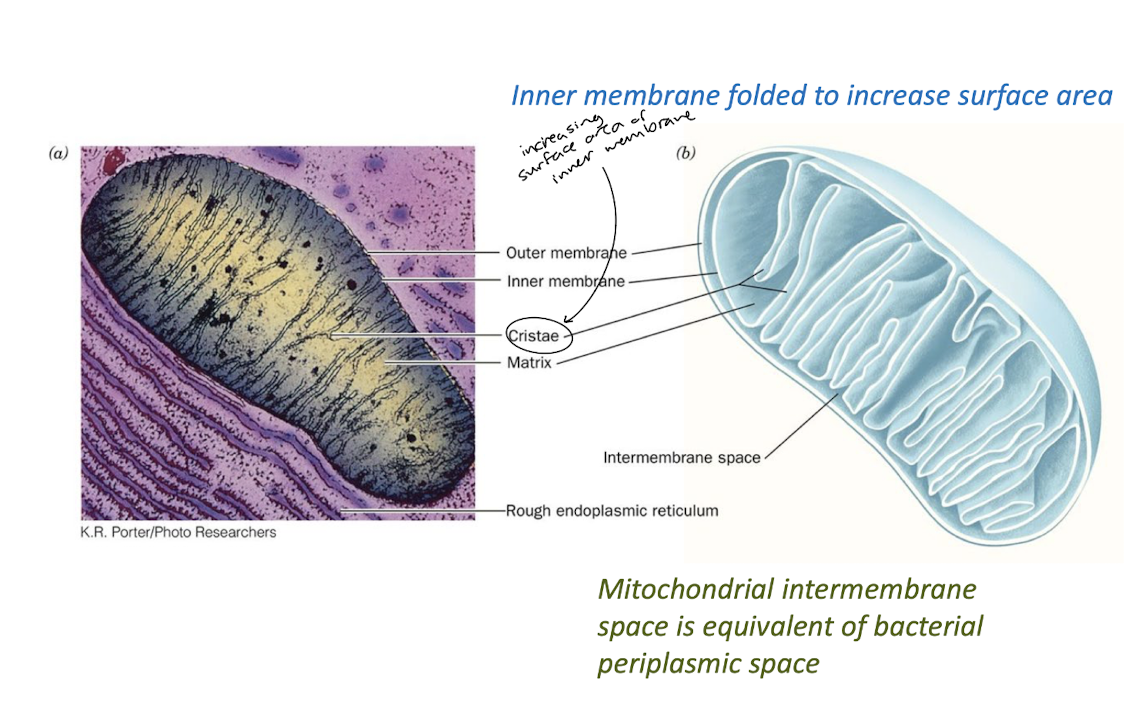
Inner and Outer Mitochondrial Membranes
Reactions in the Mitochondrion
Malate- Aspartate Shuttle
Glycerophosphate Shuttle
Checkpoint 16.1: The Mitochondrion
Draw a simple diagram of a mitochondrion and identify its structural features.
How do shuttle systems transport reducing equivalents into the mitochondria?
Learning Outcomes, 16.2: Electron Transport
Outline the components and reactions involved in electron transport.
Identify how many ATP are synthesized by the exergonic reduction of O2 by NADH.
Identify the sequence of electron carriers in the electron transport chain.
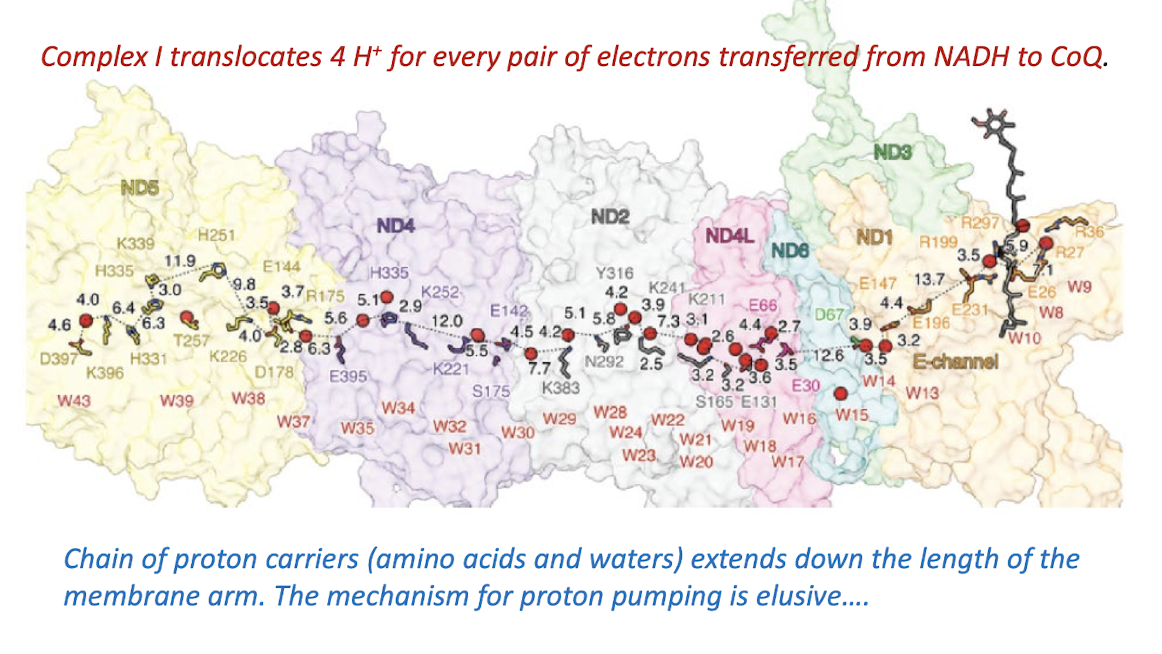
Explain how Complex I transfers the electrons from the oxidation of NADH to CoQ while increasing the transmembrane pH gradient.
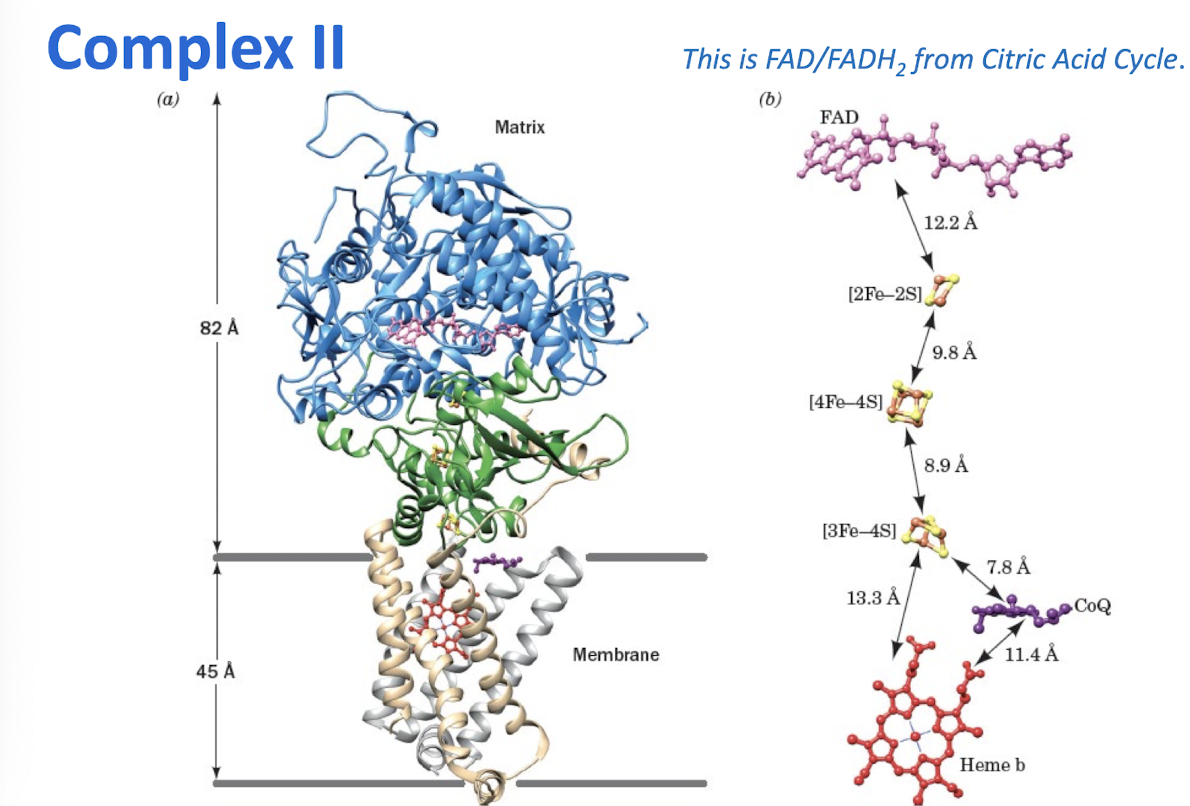
Describe how Complex II transfers electrons from succinate to the CoQ pool without contributing to the transmembrane pH gradient.
Explain how electrons in CoQH2 are transferred through Complex III to cytochrome c.
Demonstrate that the Q cycle in Complex III allows for translocation of more protons than a one-step oxidative of QH2Explain how Complex IV accepts electrons from cytochrome c to reduce O2 to H2O.
Key Concepts 16.2: Electron Transport
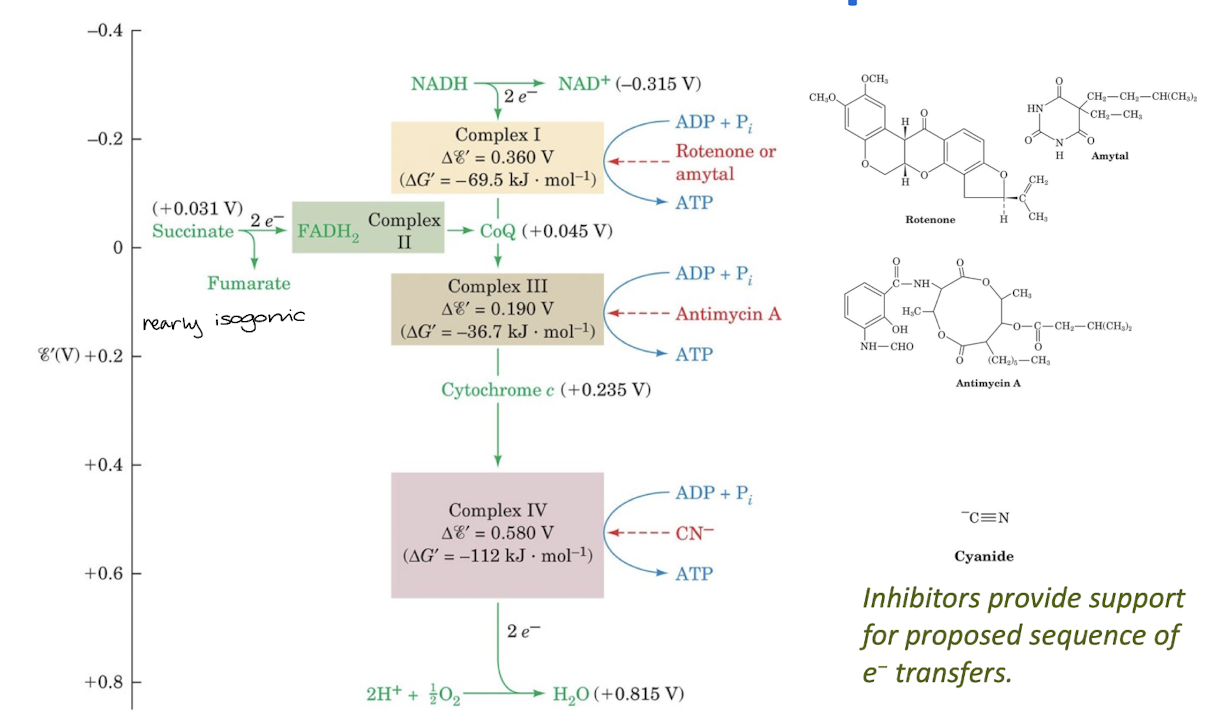
Electrons flow from redox centers with more negative reduction potential to those with more positive reduction potentials. Inhibitors have been used to reveal the sequence of electron carriers and the points of entry of electrons into the electron-transport chain.
Electron transport is mediated by one-electron carriers (Fe-S clusters, cytochromes, and Cu ions) and two-electron carriers (CoQ, FMN, FAD).
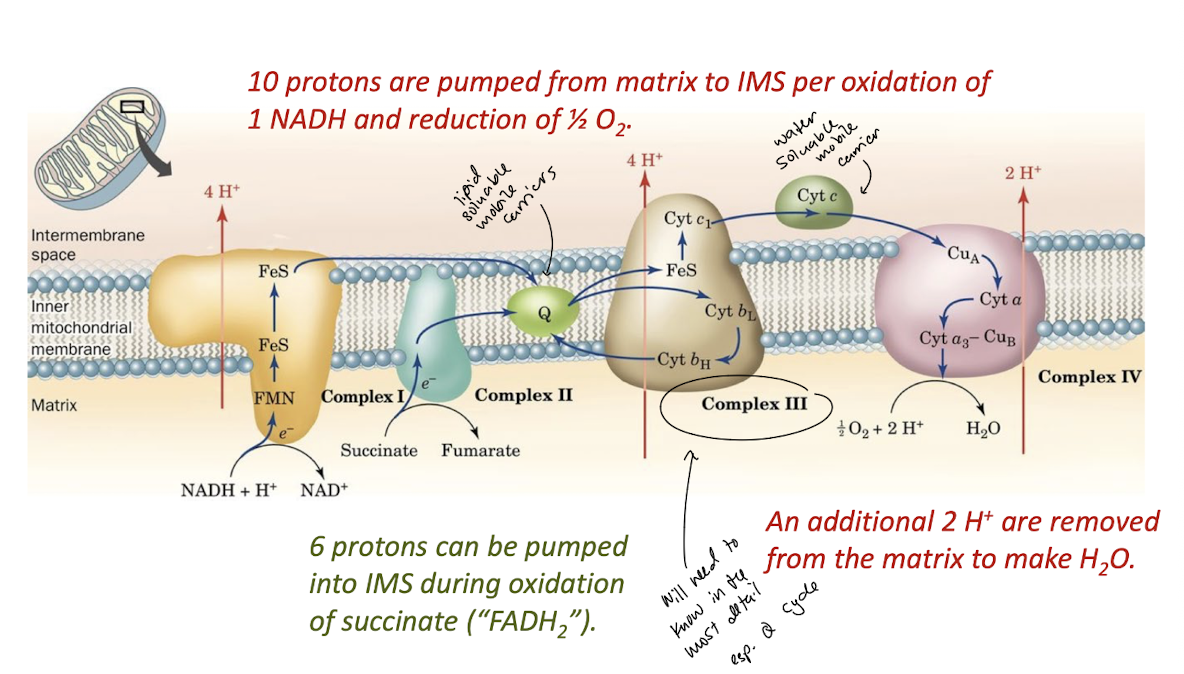
Complex I transfers two electrons from NADH to CoQ while translocating four protons to the intermembrane space.
Complex II transfers electrons from succinate through FAD to CoQ.
Complex III transfers transfers two electrons from CoQH2 to two molecules of cytochrome c. The concomitant operation of th e Q cycle translocated four protons to the intermembrane space.
Complex IV reduces O2 to 2 H2O using four electrons donated by four cytochrome c and four protons from the matrix. two protons are translocated to the intermembrane space for every two electrons that reduce oxygen.
Overview of Electron Transport
Mitochondrial Electron Transport Chain
Complex I transferring electrons
Transfers pairs of electrons, one at a time, from NADH to CoQ
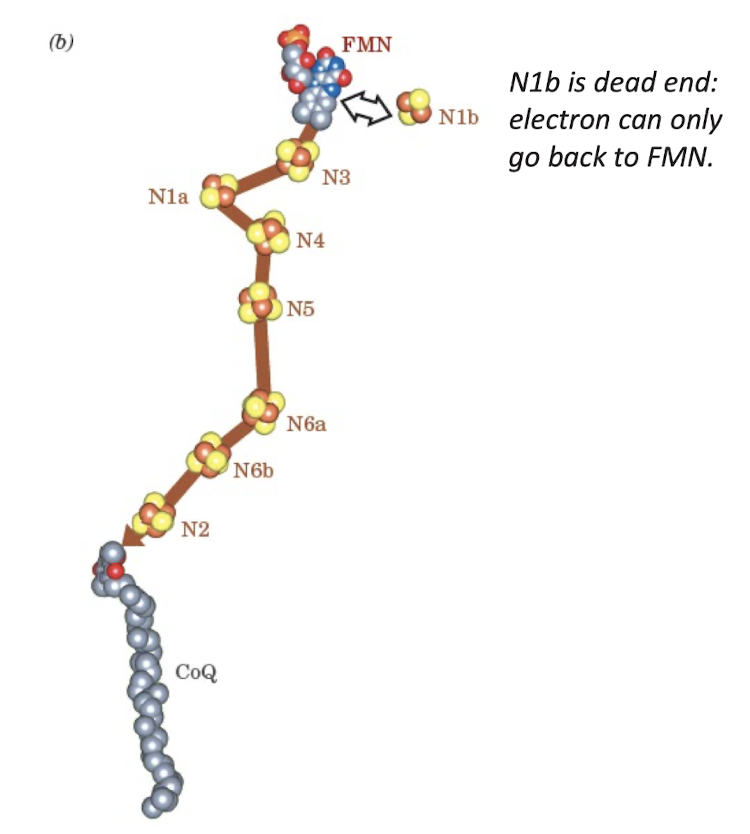
Electron Transport from FMN to CoQ
90 Å path of electron through Complex I redox groups. No jump is longer than 14 Å.
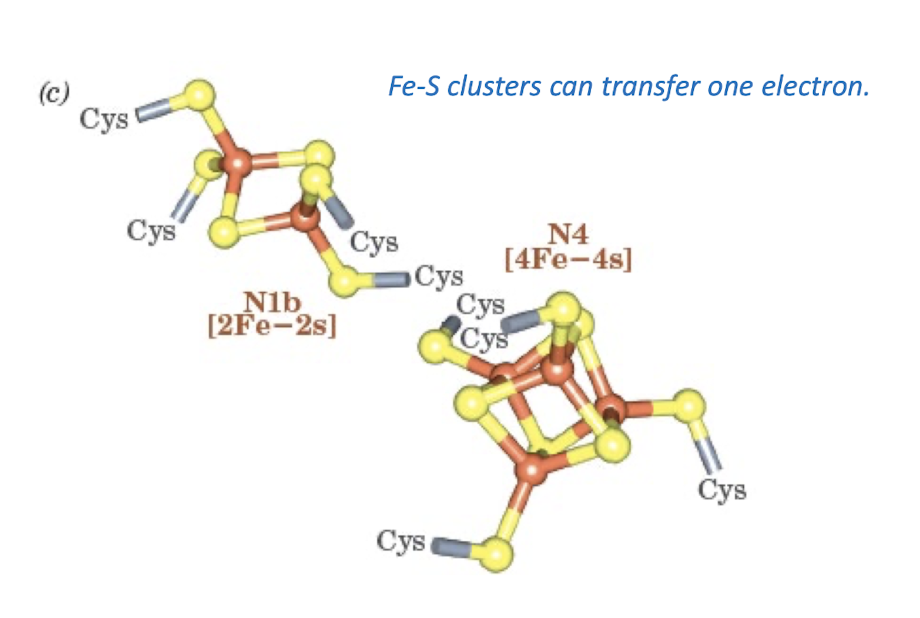
Most Common Iron- Sulfur Clusters
Fe-S clusters can transfer one electron
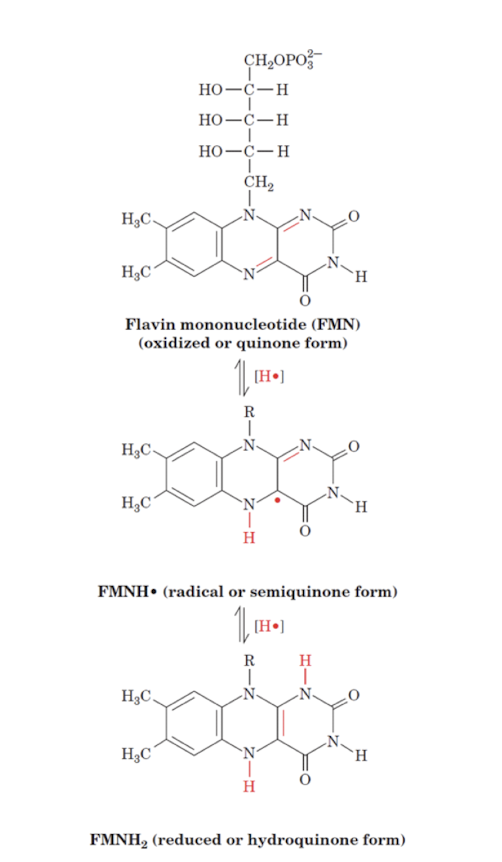
Oxidation States of FMN
Flavin is capable of both 1- and 2- electron transfers
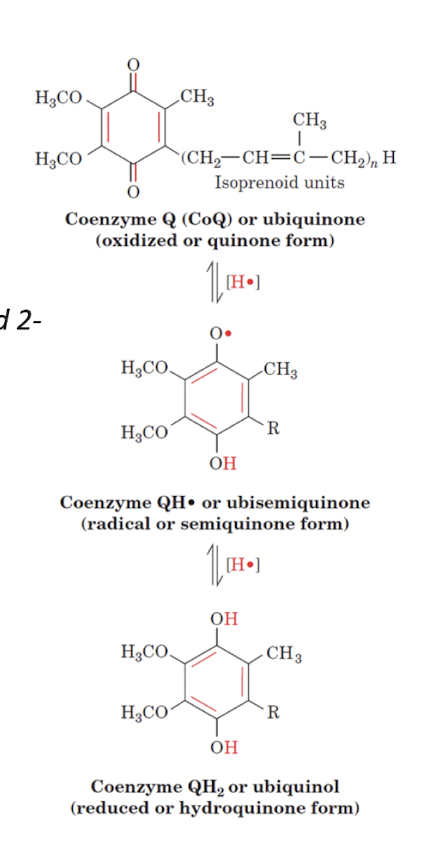
Oxidation States of CoQ
Quinone is also capable of both 1- and 2- electron transfers
A protons is also added/ lost for each electron transfer.
Hydrophilic Channel in Mitochondrial Complex I Membrane Arm
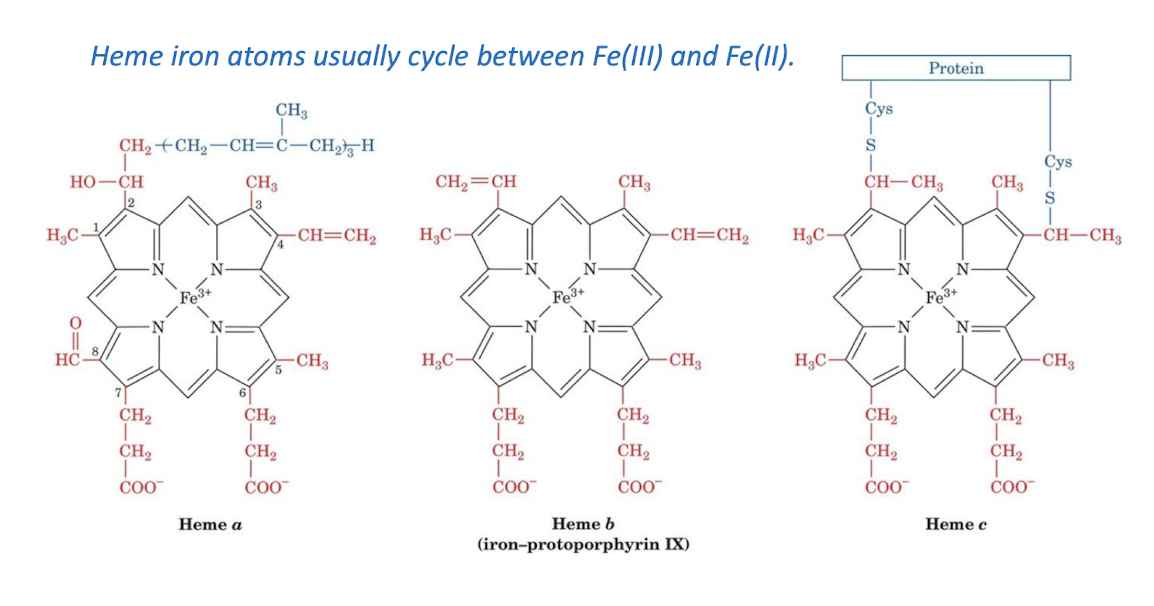
Cytochromes are electron- transport heme proteins
Complex II
Two electrons are passed from succinate to CoQ, but no protons are pumped
Complex III
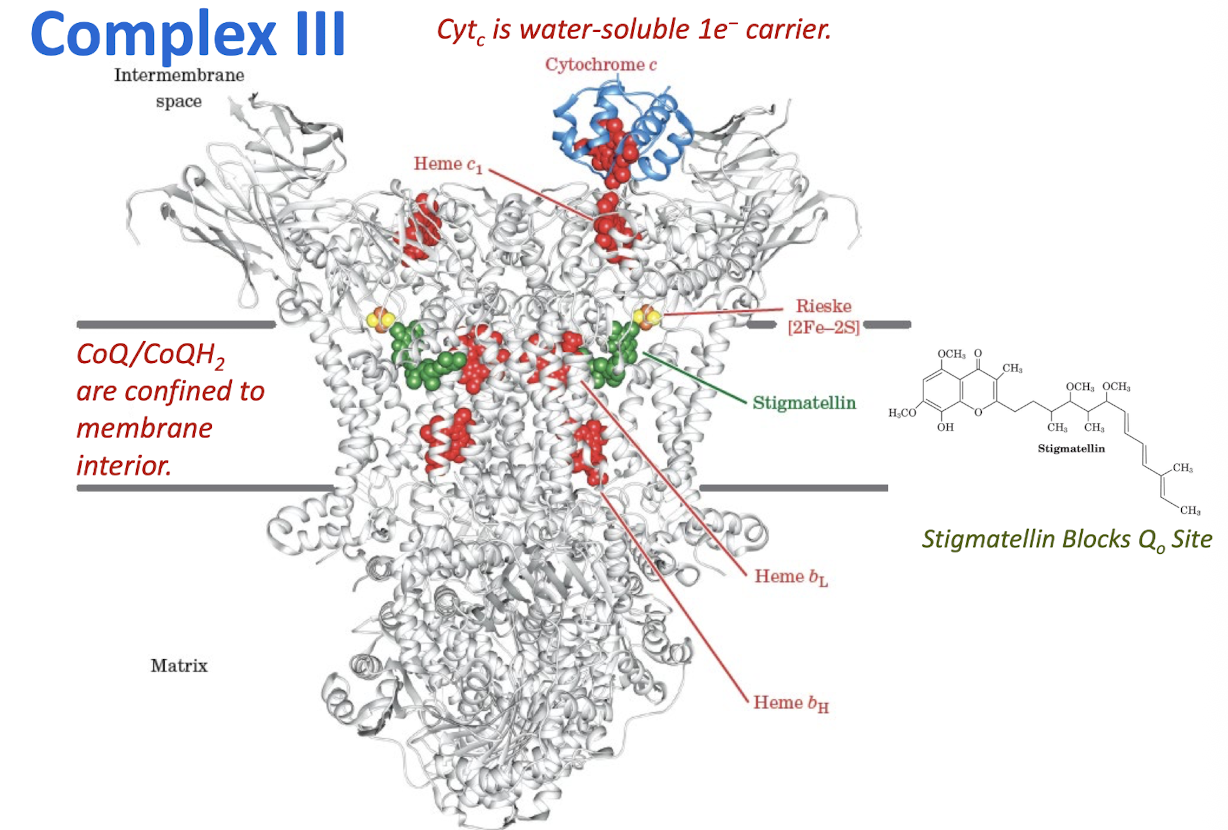
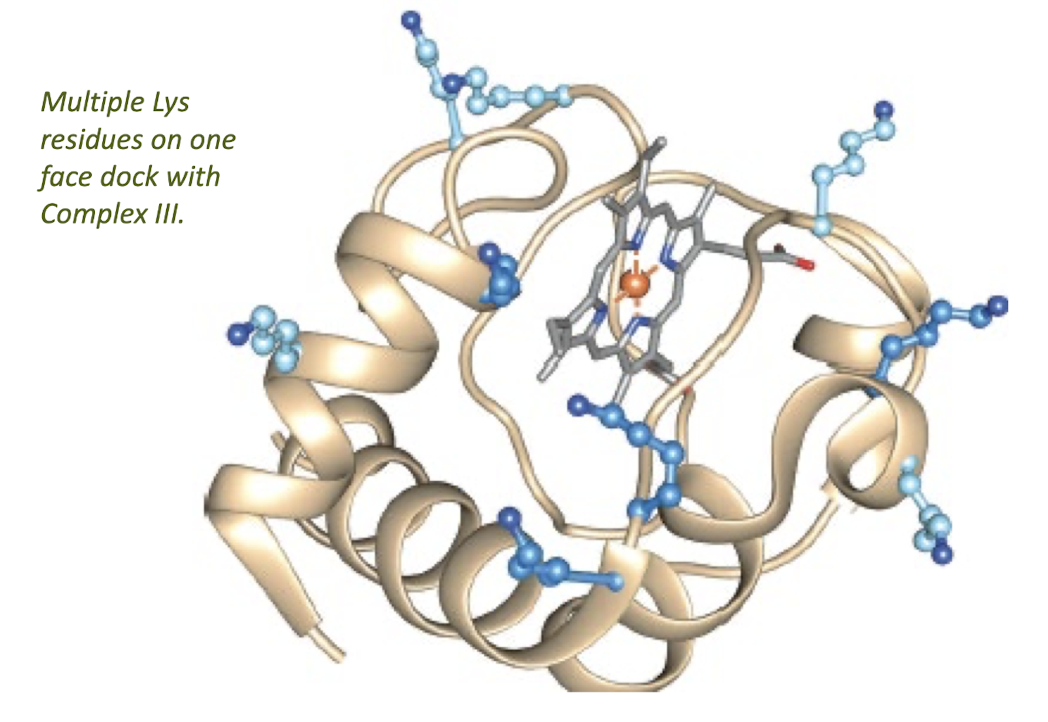
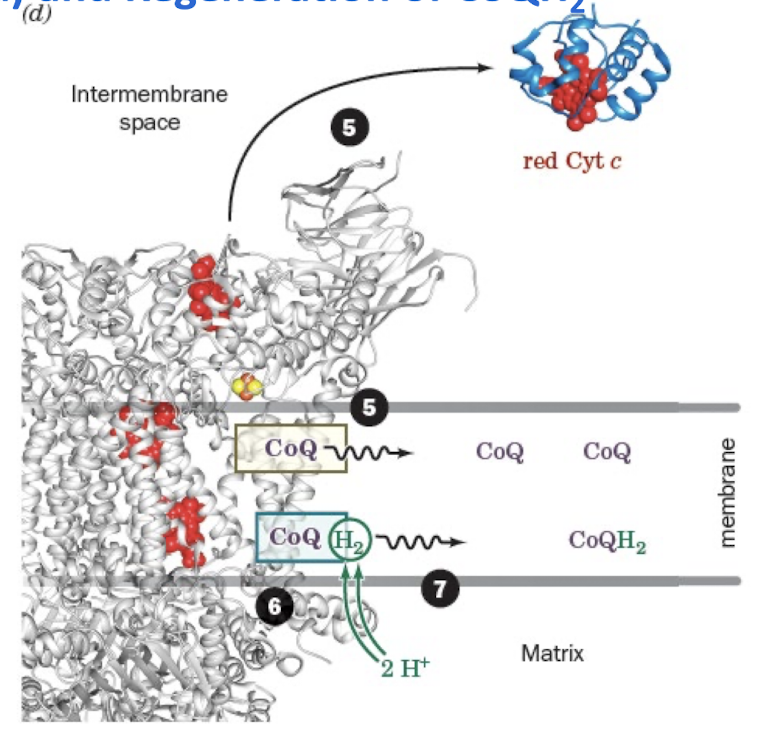
Cytochrome c
Is a Water-Soluble 1e- Carrier
reduced O2→ H2O (first time o2 is involved in aerobic catabolism after q cycle step 4)
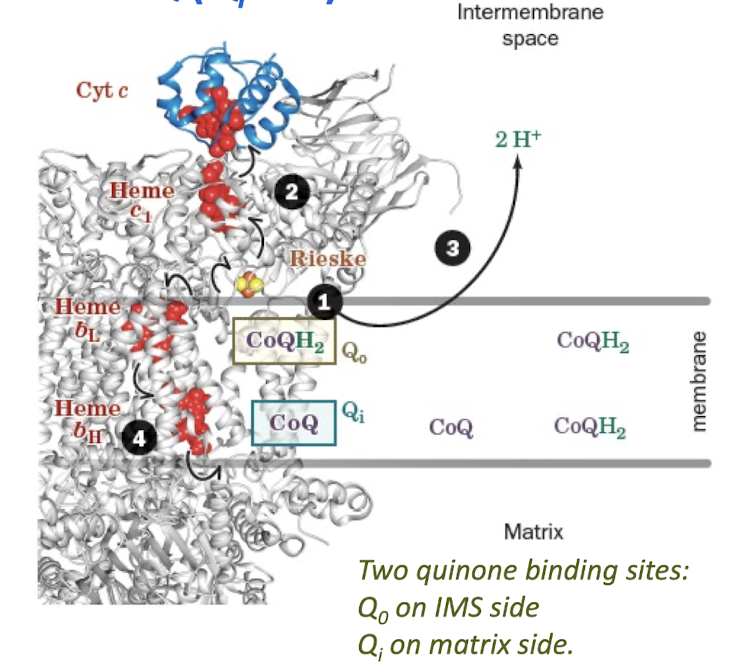
The Q Cycle Step 1:
First CoQH2 is Oxidized at Q0 site with Reduction of Cyt c and CoQ (Qi site)
Reduced CoQH2 binds at the Qo site
CoQh2 reduces the Rieske cluster, and one e- is then passed through heme c1 to a cytochrome c.
Two protons are released into the IMS, leaving a CoQ•- in the Qo site
The CoQ•- at Qo is then oxidized by passing an electron through heme bL and heme bH to reduce CoQ at Qi site.
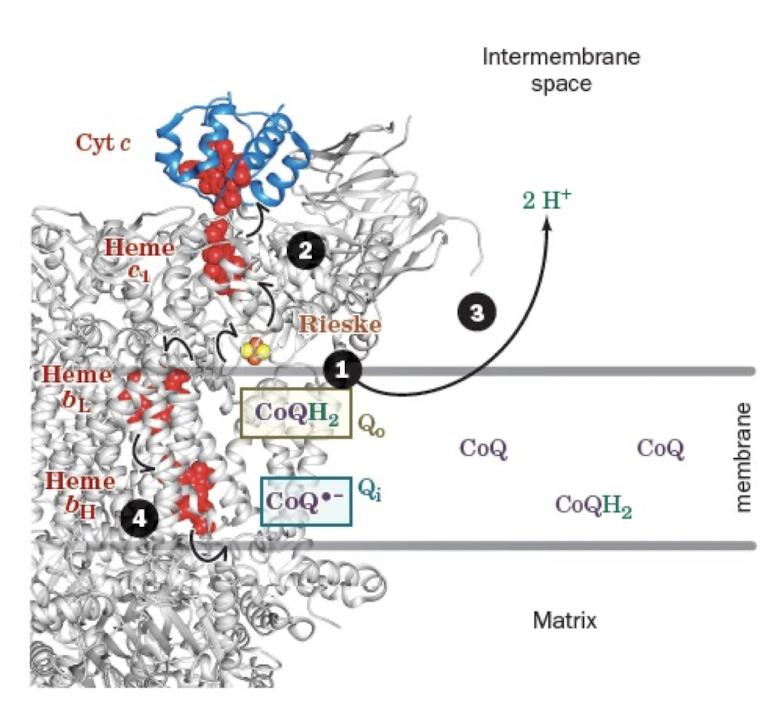
The Q Cycle Step 2:
Release of CoQ from Qo site and Cyt c
Q Cycle Step 3:
2nd CoQH2 is oxidized/ Cyt c and CoQ•- reduced
Reduced CoQH2 binds at the Qo site.
CoQH2 reduces the rieske cluster, and one e- is then passed through heme c1 to a cytochrome c.
Two protons are released into the IMS leaving a CoQ•- at Qo is then oxidized by passing an electron through heme bL and heme bH to reduce CoQ•- at Qi site.
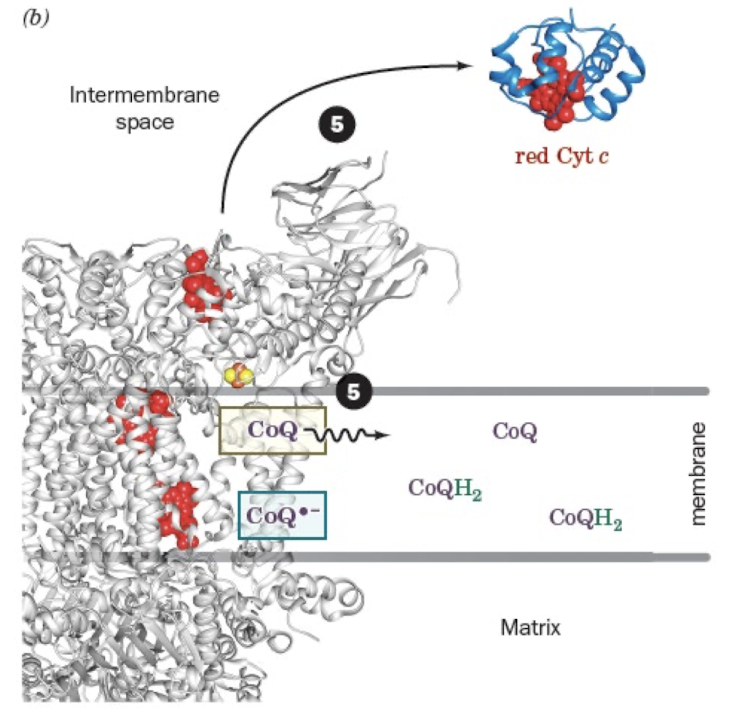
the Q Cycle Step 4:
Release of Cyt c and Regeneration of CoQH2
Release of CoQ from Qo site and Cyt c
Two H+ are taken from matrix to make CoQH2
CoQH2 is released to quinone pool.
Reduction of Oxygen
Proposed Cytochrome c Oxidase Reaction
Checkpoint 16.2: Electron Transport
What is the route followed by electron from glucose to O2?
Write the net equation for electron trnasfer from NADH to O2
For each of the electron-transport complexes on a graph showing their relative reduction potentials and indicate the path of electron flow.
How did inhibitors reveal the order of electron transport?
What are the types of prosthetic groups in Complexes I, II, III, IV? Which ones are one-electron carriers? Two electron carriers?
What does the Q cycle accomplish in each of its two rounds?
What are the different mechanism for translocating protons during electron transport?
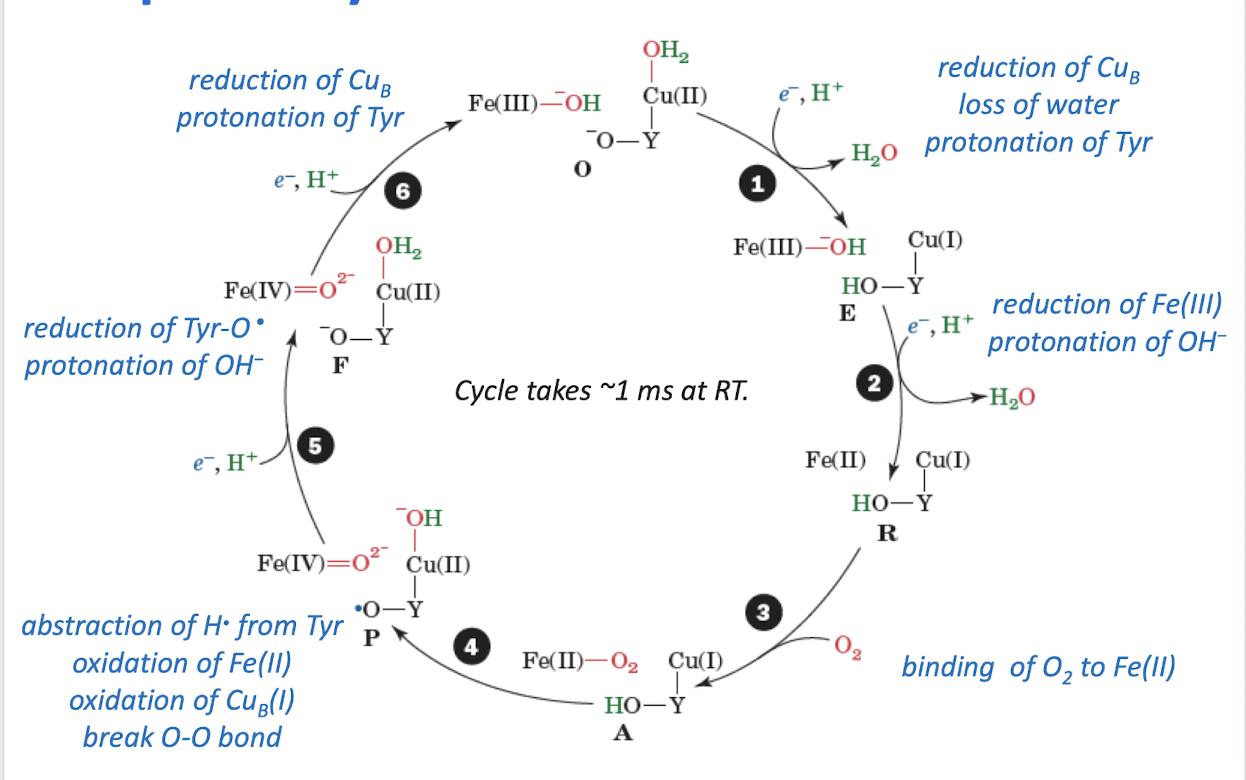
What is the significance of the Tyr radical in cytochrome c oxidase?
Learning Outcomes, 16.3: Oxidative Phosphorylation
Explain how free energy from the oxidation of metabolic fuels is used to generate ATP.
Identify the difference in pH between the mitochondrial matrix and intermembrane space as a form of potential energy used to synthesize ATP.
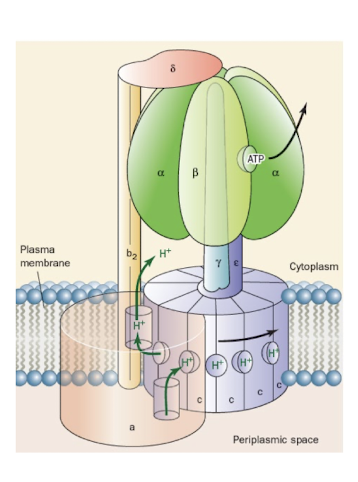
Describe the function of the F1 and F0 components of ATp synthase.
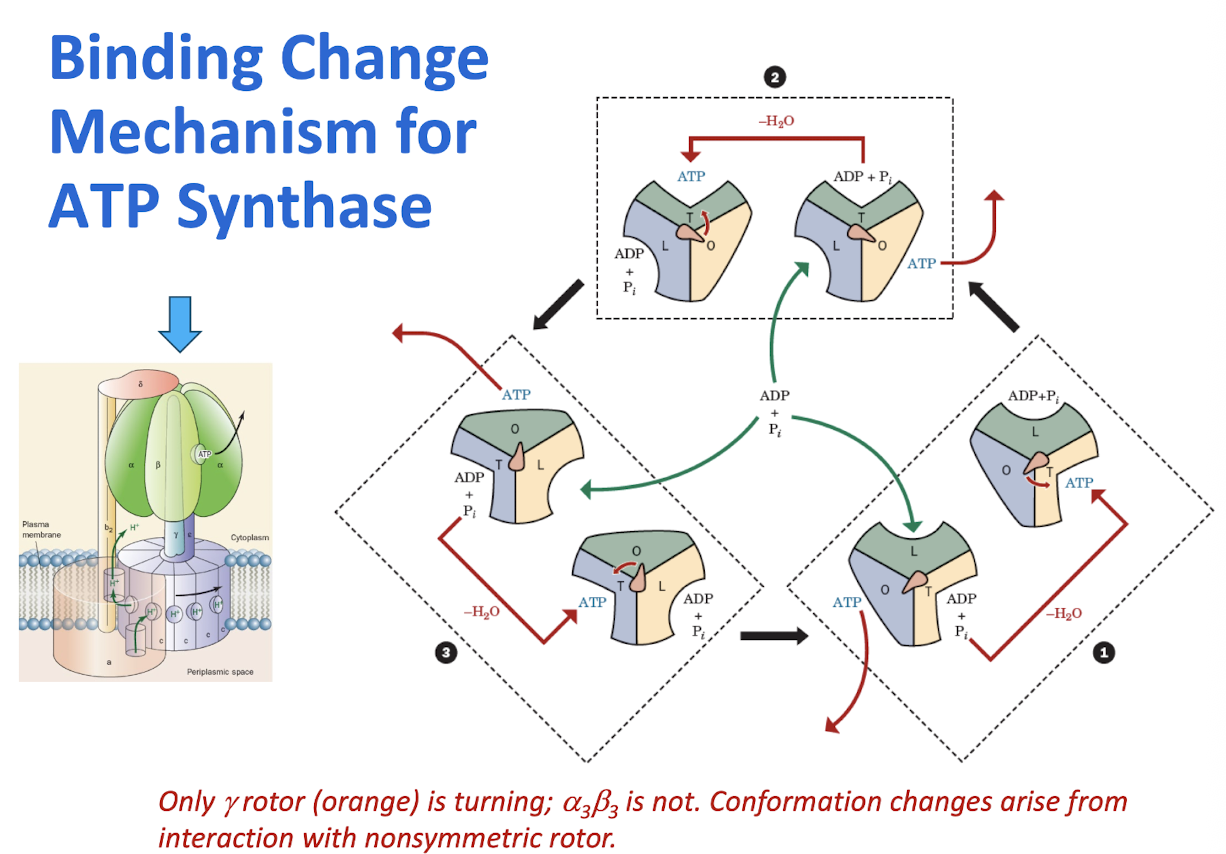
Explain how changes in conformaiton of the F1 component catalyze the reaction of ADP and Pi to make ATP.
Describe how the F0 component responds to a proton gradient across the inner membrane.
Link the turning of the c10 subunit with the conformational changes in the F1 component that catalyze ATP synthesis.
Explain that every two electrons that enter the electron-transport chain as NADH reduce one oxygen atom and produce approximately 2.5 ATP molecules.
Key Concepts 16.3: Oxidative Phosphorylation
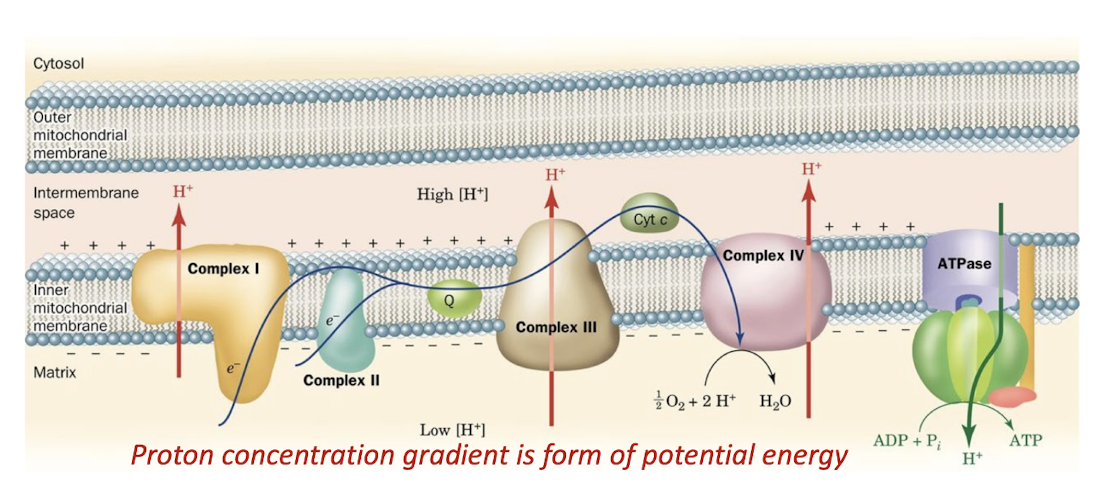
As explained by the chemiosmotic theory, protons translocated into the intermembrane space during electron transport through Complexes I, III, and IV establish an electrochemical gradient across the inner mitochondrial membrane.
The translocaiton of protons through the F0 component of ATP synthase (F1F0-ATPase) drives its F1 component to sythesize ATP from ADP+Pi via the binding change mechanism, a process that is mechanically driven by the F0-mediated rotation of F1’s γ subunit with respect to its catalytic ⍺3β3 assembly.
Coupling of Electron Transport and ATP Synthesis.
F1 Components of ATP synthase
Protrude from Mitochondrial Cristae
ATP synthase occur as pairs in creases of cristae
Structure of F1F0-ATPase
Binding Change Mechanism for ATP Synthase
Interface between c subunits and a subunit
Protons enters from channel to IMS (right), binds to Asp carboxylate.
Protonated Asp (now charge neutral) move around turnstile.
When protonated Asp reaches exit channel (left) proton is lost to matrix.
Anionic Asp side chain is eleectrostatically attracted to cationic Arg side chain.
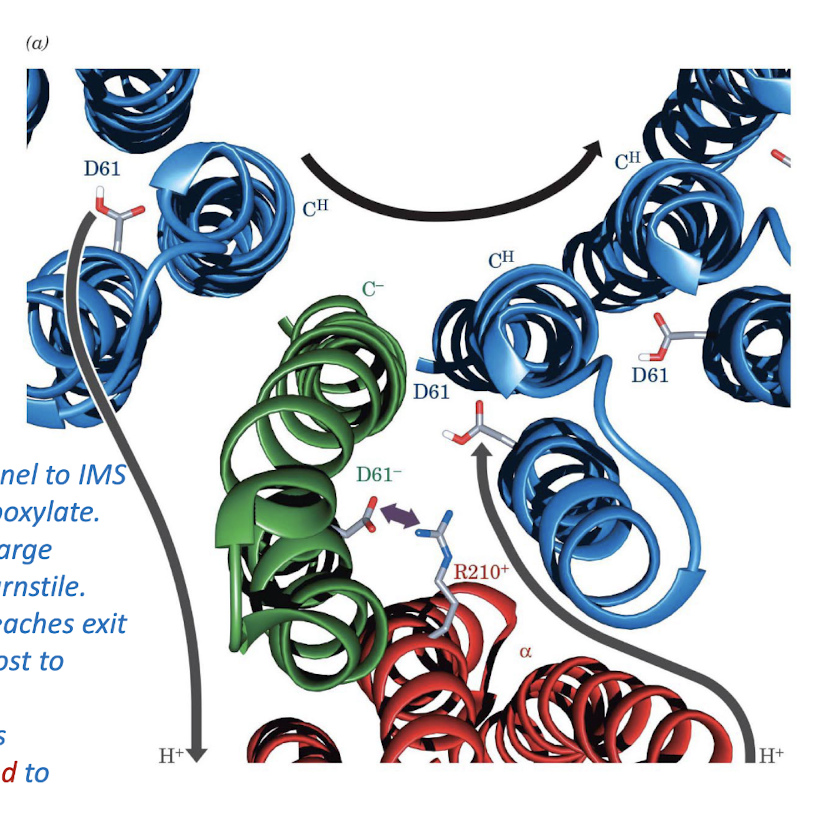
c Subunit to pH change
Conformation change due to protonation (lower pH) causes subunit to push off.
Checkpoint 16.3: Oxidative Phosphorylation
What is the chemiosmotic theory?
Why is an intact, impermeable mitochondrial membrane essential for ATP synthesis?
Describe the overall structure of the F1 and F0 components of ATP synthase. Which parts move?Which are stationary? Which are mostly stationary but undergo conformational changes?
What are the steps of the binding change mechanism?
How do protons move from the intermembrane space into the matrix? How is proton translocation linked ot ATP synthesis?
What energy transformations occur in converting the free energy of glucose to the free energy of ATP?
Learning Outcomes, 16.4: Control of Oxidative Metabolism
describe how the outcomes of oxidative metabolism are impacted by reactant concentrations, uncoupling, and reactive oxygen species.
Explain how the rate of oxidative phosphorylation is coordinated with the cell’s other oxidative pathways.
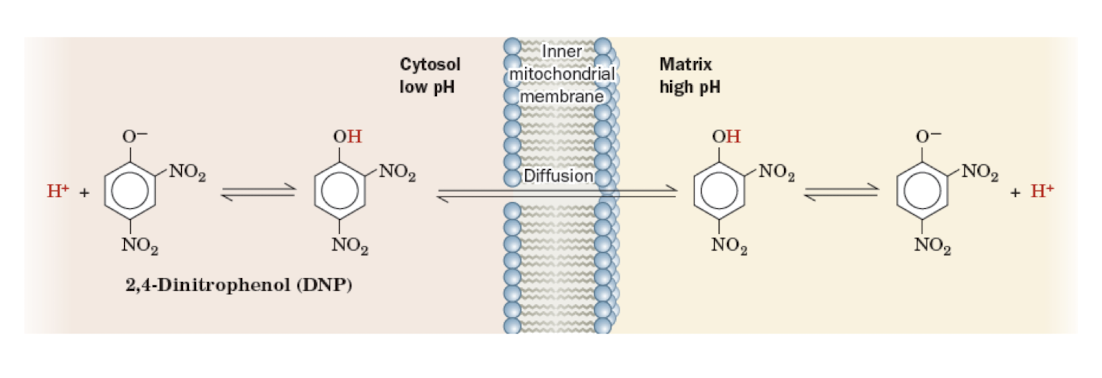
Identify how agents that dissipate the pH gradient can uncouple electron transport from ATP synthesis.
Describe the production of reactive oxygen species and their deleterious effects.
Key Concepts 16.4: Control of Oxidative Metabolism
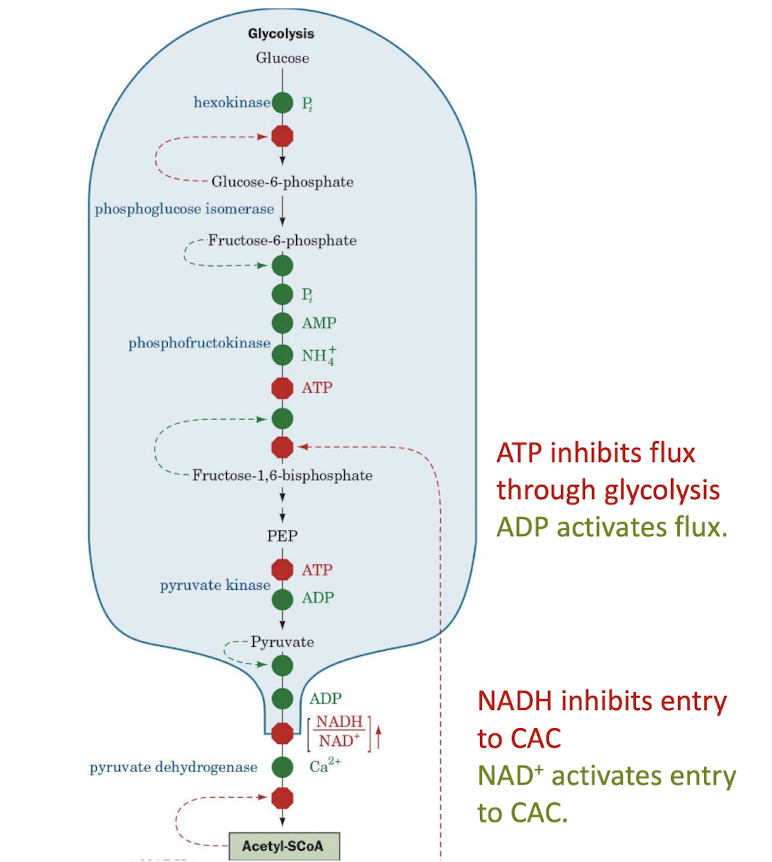
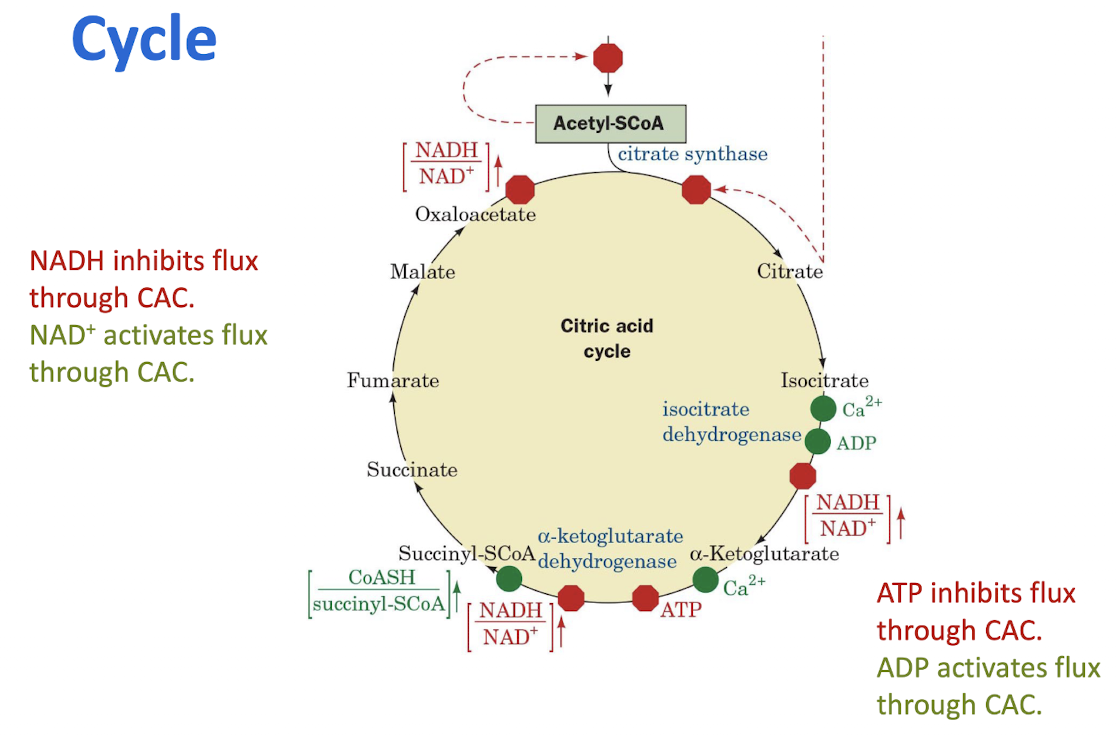
Oxidative phosphorylation is controlled by the ratio [NADH]/[NAD+] and by the ATP mass action ratio. Glycolysis and the citric acid cycle are coordinately regulated according to the need for oxidative phosphorylation.
Aerobic metabolism is more efficient than anaerobic metabolism. However, aerobic organisms must gaurd against damage caused by reactive oxygen species.
Coordinated Control of Glycolysis
Coordinated Control of the Citric Acid Cycle
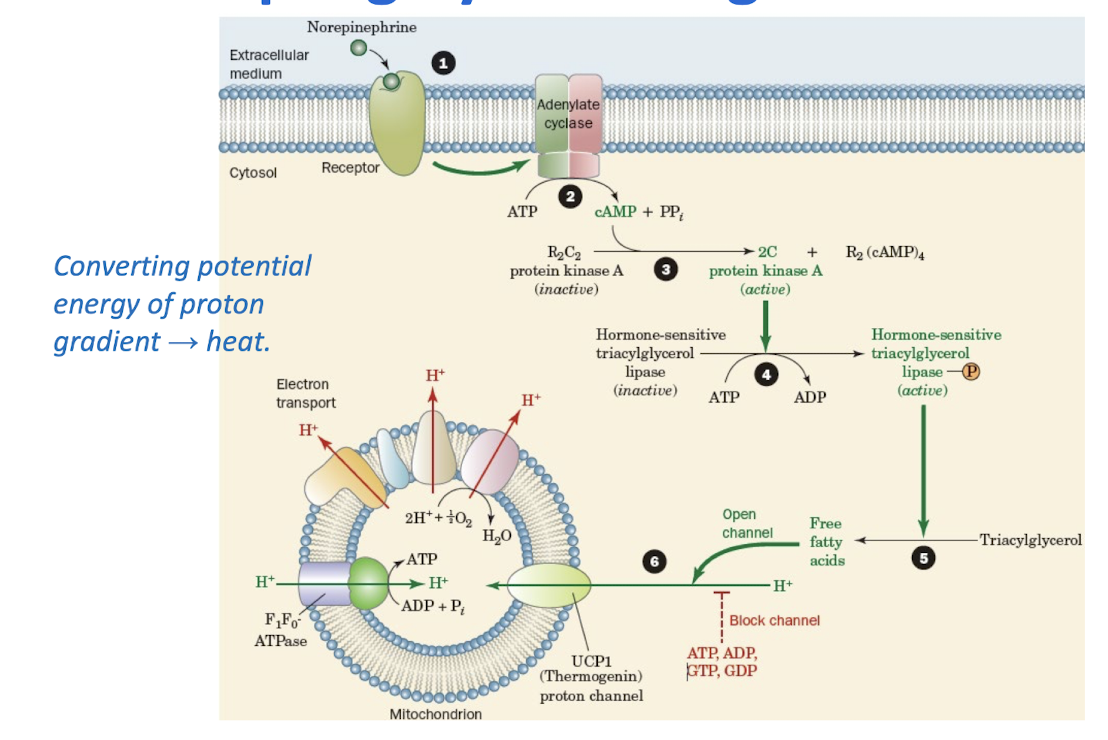
Uncoupling by Thermogenin
Artificial Uncoupling Agents
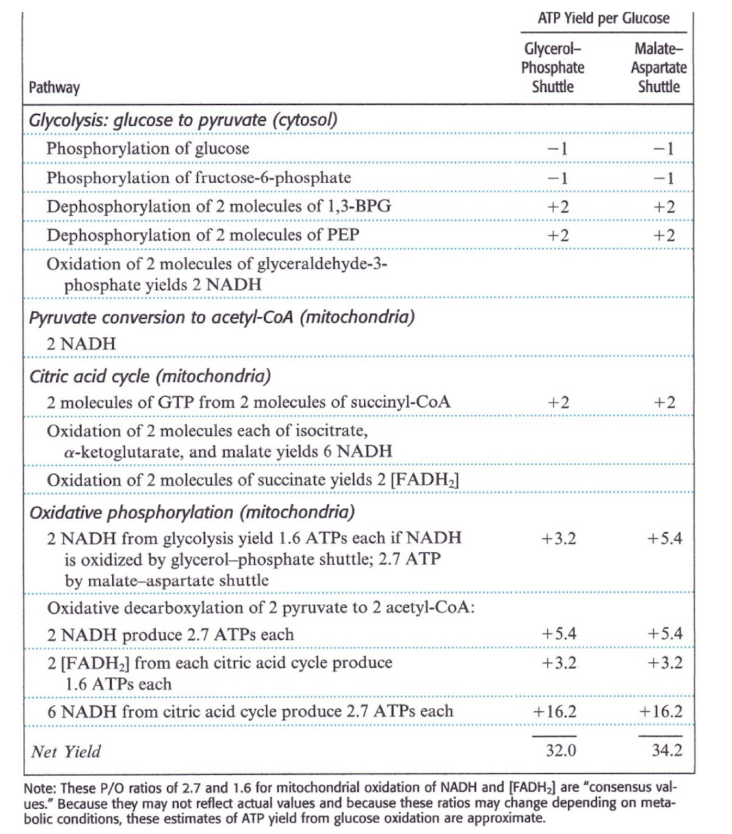
Total Yield of ATP from Glucose
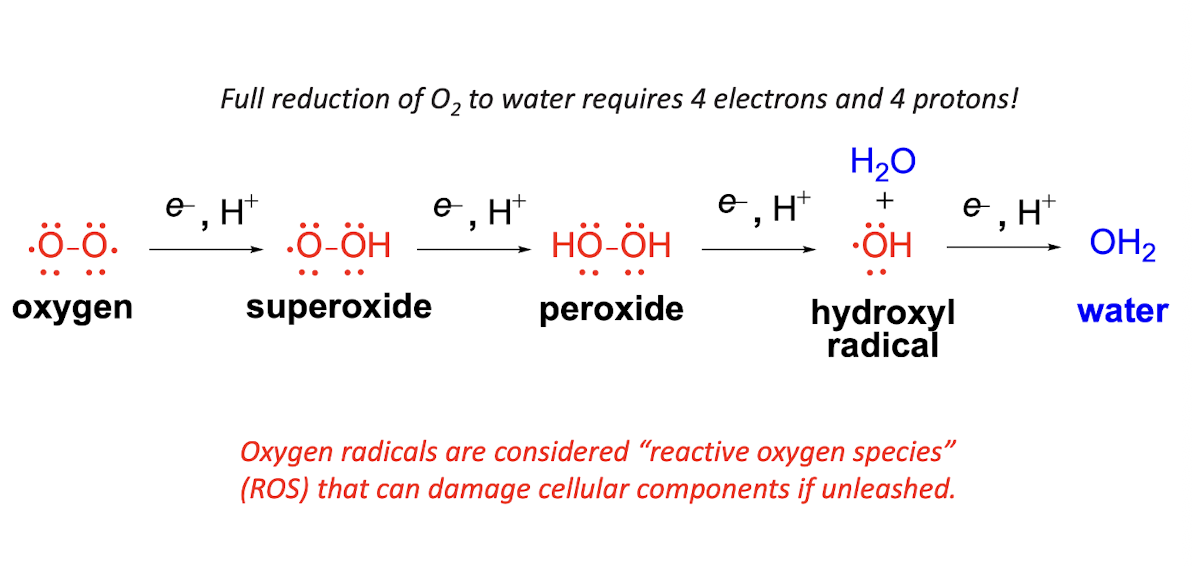
Reactive Oxygen Species Equation
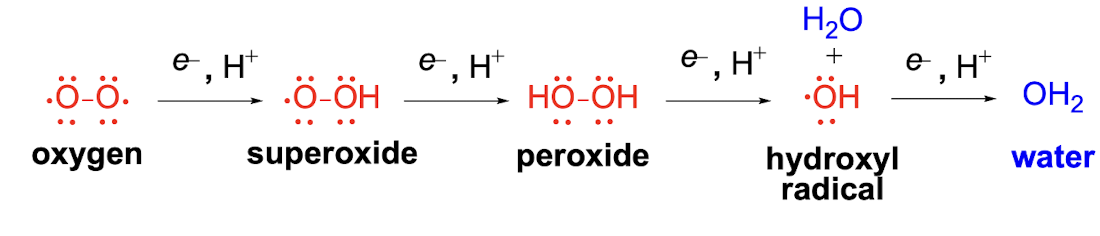
Reactive Oxygen Species Pathway
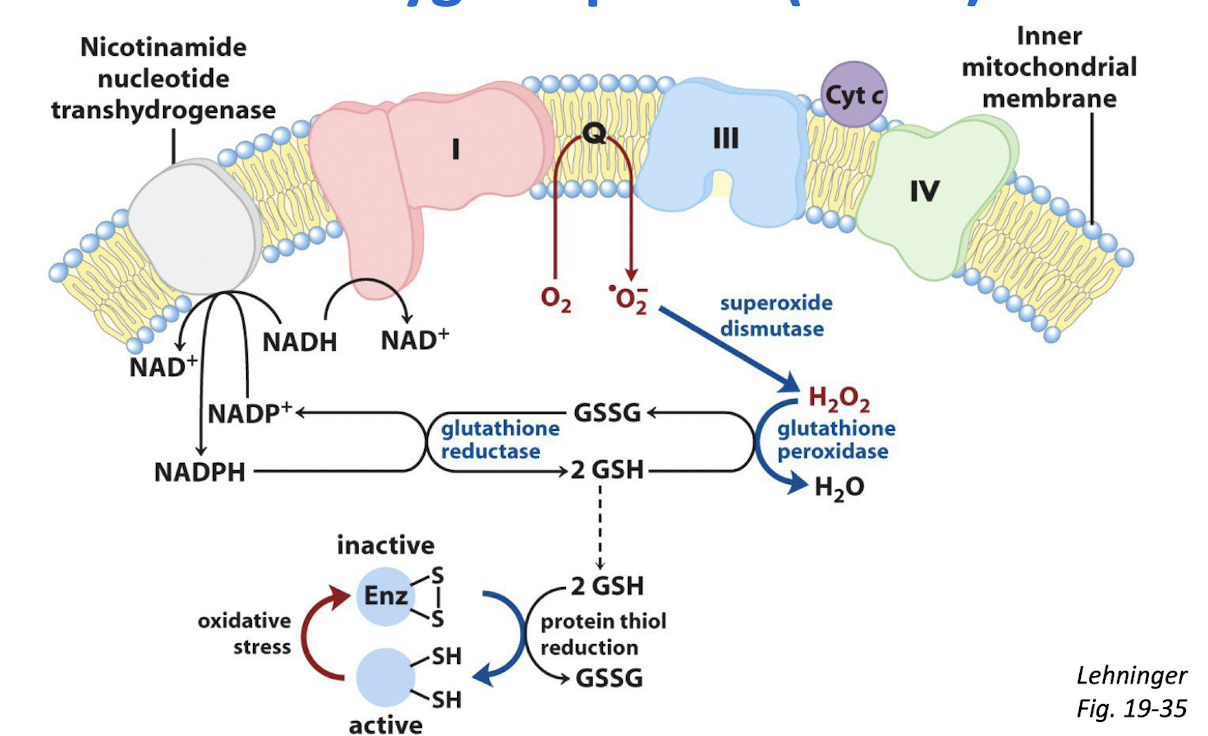
Checkpoint 16.4: Control of Oxidative Metabolism
How do the ATP mass action ratio and the IF1 protein regulate ATP synthesis?
What control mechanisms link glycolysis, the citric acid cycle, and oxidative phosphorylation?
How is oxidative phosphorylation linked to electron transport? How can the two processes be uncoupled?
What are the advantages and dis advantages of oxygen based metabolism?
How do cells minimize oxidative damage?