AP Chem Test 1B
1/13
Earn XP
Description and Tags
Topics 1.5 to 1.8
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
14 Terms
Write the ground state electron configuration for Chlorine, Cl.
1s22s22p63s23p5
Write the electron configuration of fluorine ion, F-.
1s22s22p6
Write the electron configuration for Aluminum ion, Al3+.
1s22s22p6
Write the noble gas electron configuration for scandium, Sc.
[Ar]184s23d1
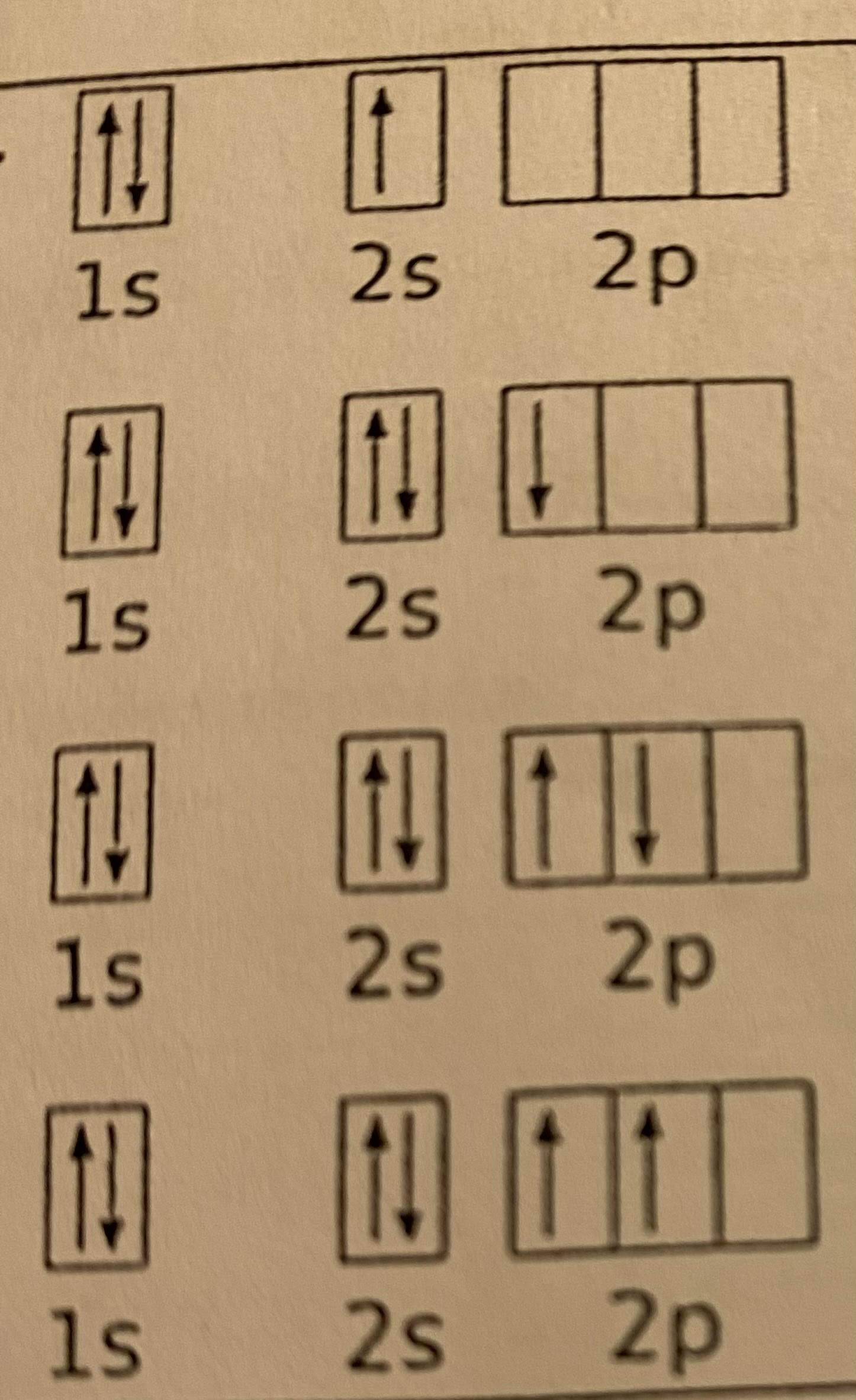
In the diagram on the right, three of the orbital diagrams are correct and one is incorrect. Identify the elements shown for each and correct the one that is wrong.
a) lithium (Li)
b) boron (B)
c) wrong; second arrow in 2p should be facing upwards; carbon (C)
d) carbon (C)
When an electron in an atom gains sufficient energy it can move to a higher energy level (further away from the nucleus). This is called an excited state. Write an electron configuration for an excited state of sodium in which one of the 2p electrons jumps up to the 3p orbital.
1s22s22p53s13p1
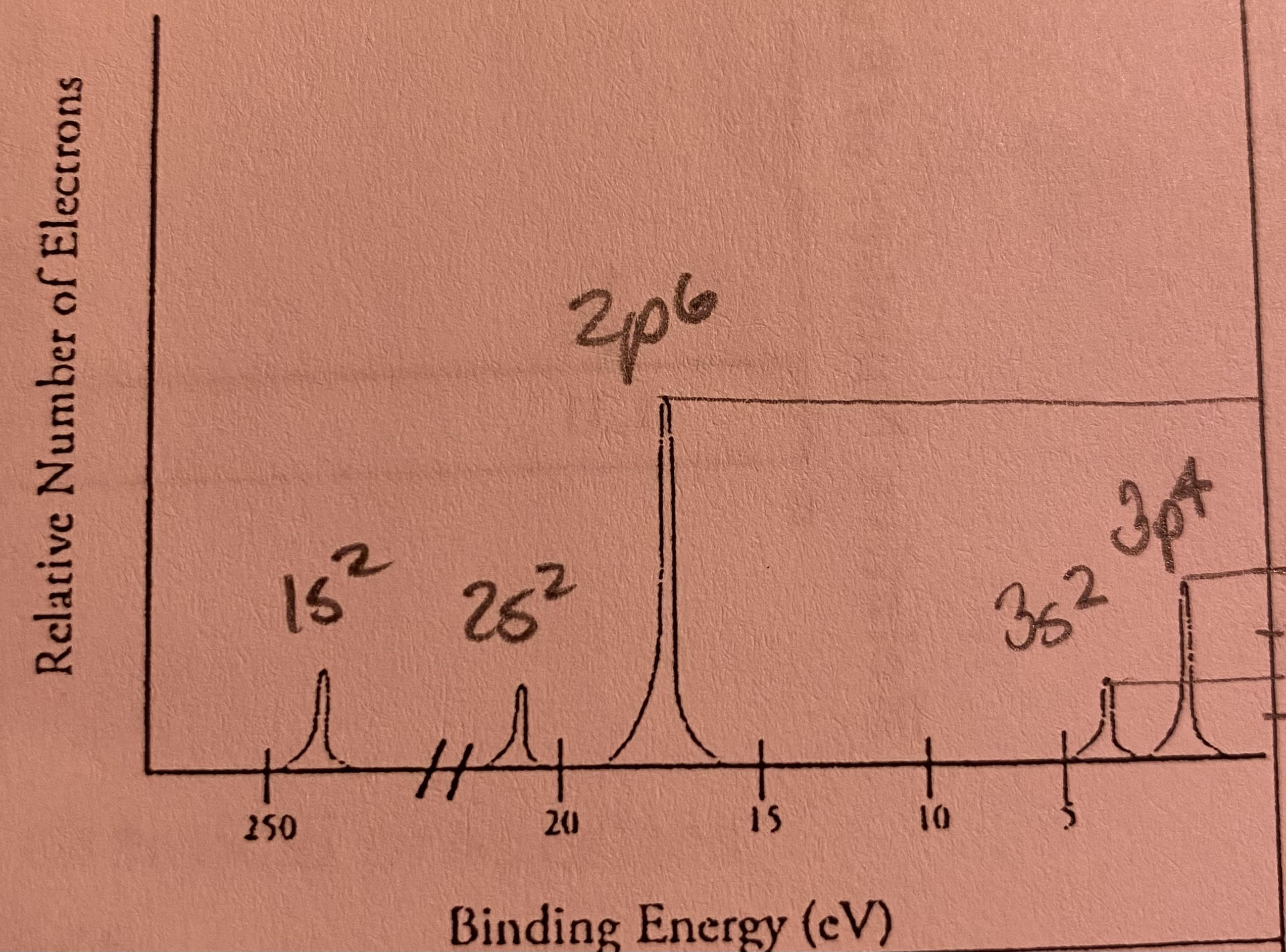
Which element is represented by the PES absorption spectra shown?
2+2+6+2+4=16 electrons=16 protons=sulfur (S)
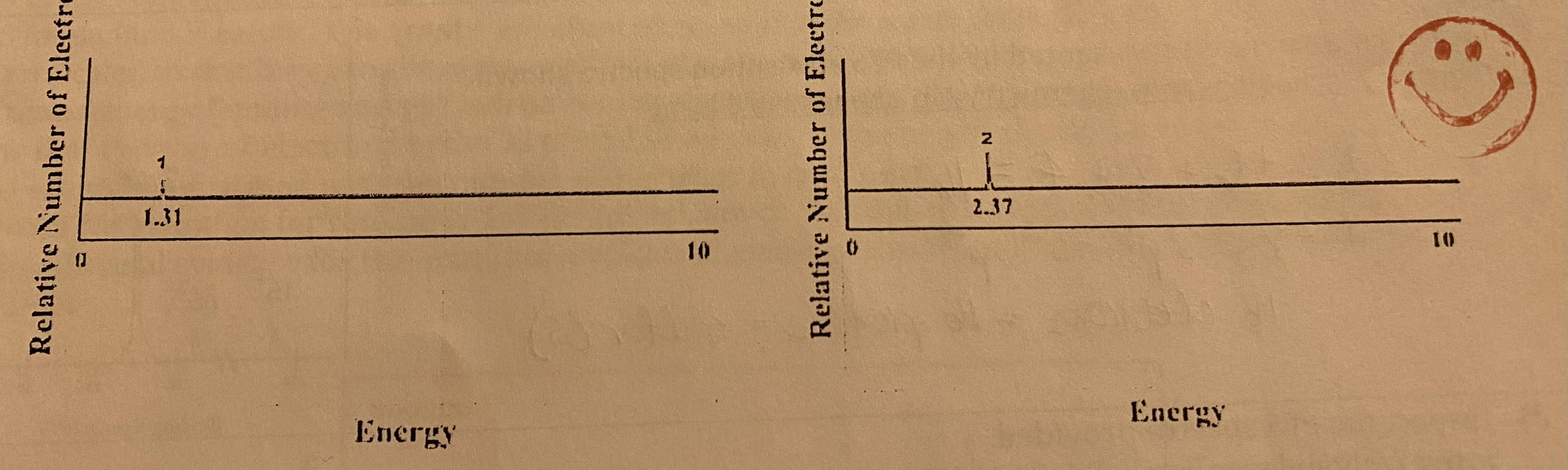
The PES spectra for hydrogen and helium are provided.
a) Label each graphs as Hydrogen or Helium
b) Explain the difference in the intensity (height) of the peaks.
c) Explain the difference in the energy of the peaks.
a) left spectra is hydrogen and right spectra is helium
b) He has two electrons, so it’s higher than hydrogen, which only has one electron.
c) He has more binding energy, so it’s farther right than H. He takes more energy to remove an electron because of the greater nuclear charge.
On the basis of their position on the periodic table determine which element in the pair would have a larger atomic radius.
a) P or S
b) Cl or Br
c) Sr or Sc
a) P
b) Br
c) Sr

Based on the successive ionization energies for the following element “X”, predict the formula that would be formed when “X” reacts with chlorine, Cl.
The jumps in enthalpy correspond to 1s22s22p63s23p1, meaning that the element is aluminum (Al). Therefore, when it reacts with chlorine (Cl), the formula would be XCl3 or AlCl3.
The first ionization energy for potassium, K, is 419 kJ/mol and the second ionization energy for calcium, Ca, is 1145 kJ/mol. Using concepts from this unit explain why they are different even though they are isoelectric (have the same number of electrons).
Ca has more protons, so there is a stronger attraction.
Element X has an electron configuration of 1s22s22p63s1, while element Z has an electron configuration of 1s22s22p5.
a) Which element would have greater first ionization energy?
b) Which element would have a larger radius?
c) Which element would have higher electronegativity?
d) Which element would form an ion that has a larger radius?
e) Which element would release more energy when it gains an electron?
a) Z
b) X
c) Z
d) Z
e) Z
Which of the following has the same number of electrons as Cl-1?
a) F-1
b) S
c) Al3+
d) K+
d) K+ because it has the same number of electrons as Cl-1, which is 18.
KCl dissolves in water, forming a solution able to conduct electricity. Which of the following would behave similarly?
a) PbCl2
b) LiK
c) LiCl
d) SrCl2
c) LiCl