Chem 1C Midterm 2 - OCHEM functional groups
1/8
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
9 Terms
alkanes
properties:
IMFs - LDF
low BP, high VP
insoluble in water
naming:
#C-branchnamebasename’ane’
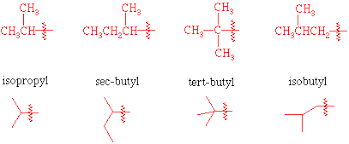
branched alkanes
isopropyl
little fork
isobutyl
little fork + methyl
sec-butyl
wiggly chain
tert-butyl
cross
alcohols
oxygen is sp3, bent molecule
properties:
IMFs: LDF, D/D, H-bond
higher BP than alkanes
polar, soluble in water
can lose -OH or H (amphoteric)
naming:
like alkane, but replace ‘e’ with ‘ol’
give smallest # near to OH group
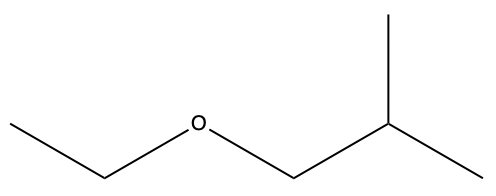
ethers
oxygen is sp3, bent molecule
properties:
IMFs: LDF, D/D, moderate BP
polar for small
naming:
IUPAC (formal)
name longest chain like alkane
name shorter part, branch with o, end in ‘oxy’
C# for branch
common
list substituents A→Z
then ‘ether’
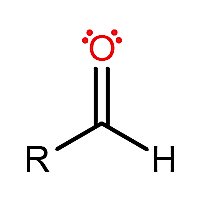
aldehydes
carbon is sp2, trigonal planar
properties:
IMFs: LDF, D/D
polar for small
naming:
like alkane, replace ‘e’ with ‘al’
ketones
carbon is sp2, trigonal planar
properties:
IMFs: LDF, D/D, moderate BP
polar for small
naming:
end in ‘one’

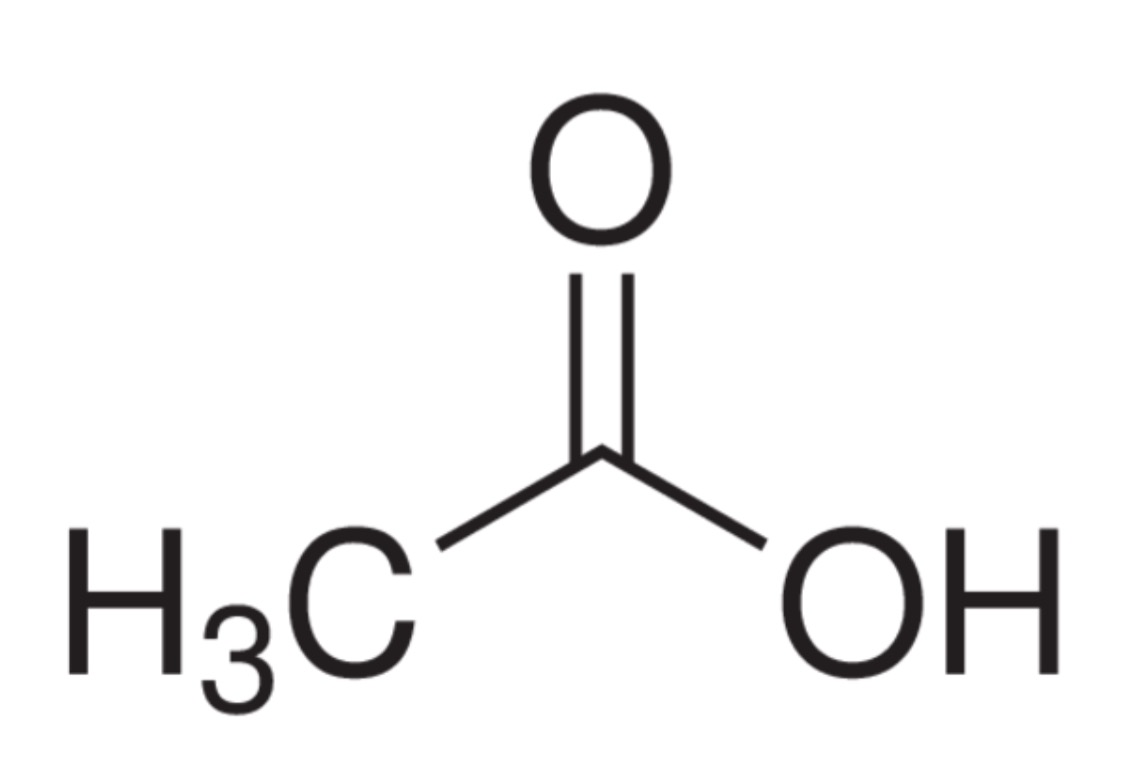
carboxylic acids
properties:
IMFs: LDF, D/D, H-bond
higher BP
amphoteric
naming:
end in ‘oic acid’
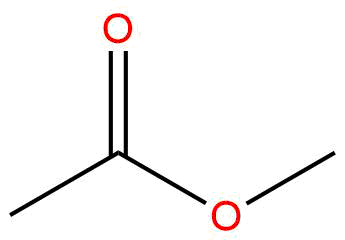
esters
properties:
IMFs: LDF, D/D
usually sweet
naming:
first count R’ ends in ‘yl’
second count R ends in ‘oate’
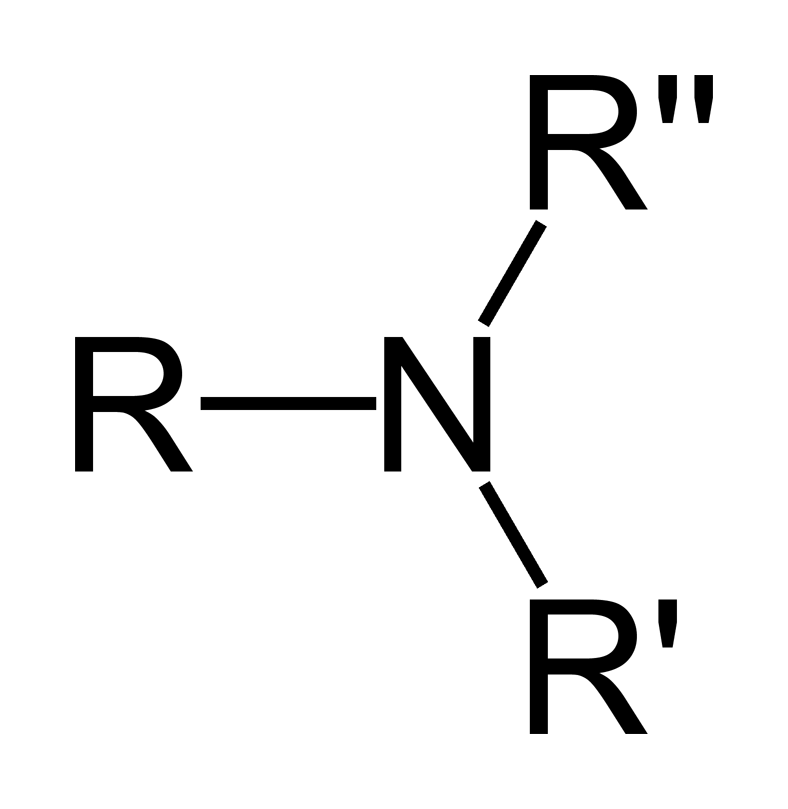
amines
properties:
contain nitrogen
primary, secondary, and tertiary (shown)
naming:
formal
longer chain like alkanes, but replace ‘e’ with ‘amine’
C# where attached to N
shorter chains A→Z and in ‘yl’
use ‘N’ instead of # if attached to N
common
list substituents A→Z then ‘amine’