Innate Immune Mechanisms (MT1/2)
5.0(1)
Card Sorting
1/29
Earn XP
Description and Tags
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
30 Terms
1
New cards
Describe: Inflammation
An innate, non-specific, broad immune response involving increased vascular permeability, cellular recruitment, cellular proliferation and metabolism (activity) that brings leukocytes and plasma proteins to the site of infection.
2
New cards
Describe: Cytokines
Cytokines are small proteins released by cells, usually in response to a stimulus, that induce a response in a target cell by binding to an appropriate cytokine receptor. Can result in activation, repression, inflammation, anti-inflammatory responses, etc...
3
New cards
What are the four families of cytokines?
Interleukins
Hematopoietins
Tumor-necrosis Factor
Interferons
Hematopoietins
Tumor-necrosis Factor
Interferons
4
New cards
Describe: Chemokines
Chemoattractant cytokines that induce cellular adhesion or directional cell migration in response to a gradient of the chemokine (road maps that tell cells where to go)
5
New cards
What are the three families of chemokines?
Are classified into families based on the location of 2
conserved cystine (C) residues.
CCL (C groups next to each other)
CXCL (1 amino acid between)
CX3CL (3 amino acids between)
conserved cystine (C) residues.
CCL (C groups next to each other)
CXCL (1 amino acid between)
CX3CL (3 amino acids between)
6
New cards
Describe the process of inflammation
Bacteria trigger macrophages to released cytokines and chemokines. Vasodilation and increased permeability cause redness, heat, and swelling. Inflammatory cells migrate into tissue releasing inflammatory mediators that cause pain.
7
New cards
What are the 4 changes to the vasculature caused by the mediators of inflammation?
1) vascular dilation → increased blood flow (heat, redness)
2) endothelium expression of “adhesion molecules” →allows for recruitment of leukocytes
3) vascular leakage → fluid and plasma proteins (complement, antibodies) enter the tissue (swelling, pain)
4) clotting → blocks the vessels preventing pathogen spread
2) endothelium expression of “adhesion molecules” →allows for recruitment of leukocytes
3) vascular leakage → fluid and plasma proteins (complement, antibodies) enter the tissue (swelling, pain)
4) clotting → blocks the vessels preventing pathogen spread
8
New cards
What is the benefit of closing blood vessels?
Keeps bacteria out of the bloodstream (bacteria in bloodstream cause sepsis and organ infection)
9
New cards
What are the dangers of excessive inflammation?
- Too much, or uncontrolled inflammation is detrimental to the host leading to organ failure and death
- Often, in the case of sepsis, the infection itself can be controlled, but not the host response.
-Systemic changes to the vasculature leads reduced blood flow through critical organs, clotting occludes blood vessels, inflammation leads to tissue damage……
- Often, in the case of sepsis, the infection itself can be controlled, but not the host response.
-Systemic changes to the vasculature leads reduced blood flow through critical organs, clotting occludes blood vessels, inflammation leads to tissue damage……
10
New cards
What are the fundamental problems with getting innate immune cells to a site of infection?
1. cells circulate through out the body and need to find the specific site of infection
2. cells are flying past any given spot, carried by the blood flow
3. cells need to get out of the blood vessels and into the tissue
4. once in the tissue leukocytes need to be able to hone-in on the pathogen…
2. cells are flying past any given spot, carried by the blood flow
3. cells need to get out of the blood vessels and into the tissue
4. once in the tissue leukocytes need to be able to hone-in on the pathogen…
11
New cards
Define: Cellular Recruitment
A tightly regulated, multistep process to recruit cells to a site of infection
12
New cards
What are the steps of the Leukocyte Recruitment Cascade, describe each.
1. Tethering - When circulating cells first contact an activated endothelium. Selectins bind to the mucin CAMS of the leukocyte and form catch bonds.
2. Rolling - Weak interactions between selectins
and mucin CAMS are constantly letting go
and re-forming, serves to slow cell down.
3. Activation - Chemokines binding to their appropriate receptor, and activate the integrin
4. Adhesion - Integrins bind to Ig-superfamily CAMs this causes the leukocyte to flatten out and crawl along the endothelium looking for a way out of the vessel
5. Transmigration - Leukocytes squeeze between or in some cases pass directly through endothelial cells to enter the
tissue below.
2. Rolling - Weak interactions between selectins
and mucin CAMS are constantly letting go
and re-forming, serves to slow cell down.
3. Activation - Chemokines binding to their appropriate receptor, and activate the integrin
4. Adhesion - Integrins bind to Ig-superfamily CAMs this causes the leukocyte to flatten out and crawl along the endothelium looking for a way out of the vessel
5. Transmigration - Leukocytes squeeze between or in some cases pass directly through endothelial cells to enter the
tissue below.
13
New cards
Which adhesion molecules are involved in rolling and tethering?
Mucin CAMs and Selectins
14
New cards
Which adhesion molecules are involved in adhesion and translocation?
Integrins and Ig-superfamily CAMs
15
New cards
Why don’t all leukocytes get recruited to a site of infection
Differential expression of adhesion molecules
Differential expression chemokine receptors
Essentially the immense variety in possible combinations of leukocyte specificity
Differential expression chemokine receptors
Essentially the immense variety in possible combinations of leukocyte specificity
16
New cards
Define: Catch Bonds
Bonds between selectins and mucin CAMs that only form in flow conditions (won't form without movement or a "pull")
17
New cards
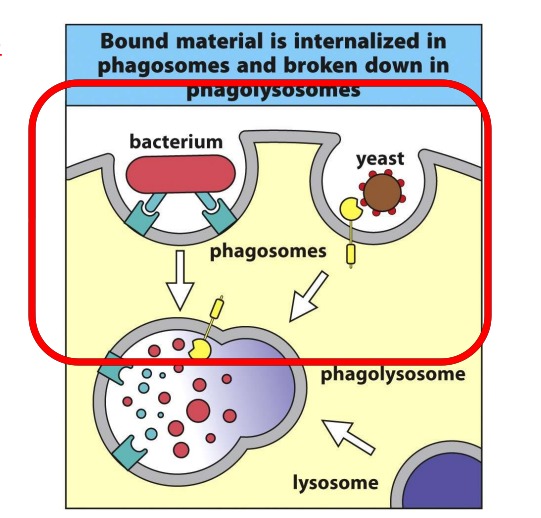
Describe: Phagocytosis
A process whereby innate immune cells “eat” pathogens
Used as the principle mechanism for macrophages, DC and neutrophils
Involves enclosing the pathogen in a membrane-bound vesicle called a phagosome.
The phagosome is then fused with other vesicles called lysosomes resulting in the exposure of the pathogen to a mileau of anti-microbial peptides and cytotoxic enzymes
This fused vesicle is known as a phagolysosome
Used as the principle mechanism for macrophages, DC and neutrophils
Involves enclosing the pathogen in a membrane-bound vesicle called a phagosome.
The phagosome is then fused with other vesicles called lysosomes resulting in the exposure of the pathogen to a mileau of anti-microbial peptides and cytotoxic enzymes
This fused vesicle is known as a phagolysosome
18
New cards
Simply enclosing a pathogen in a phagosome is not sufficient
to kill the organism. What other mechanisms are carried out by phagocytes?
to kill the organism. What other mechanisms are carried out by phagocytes?
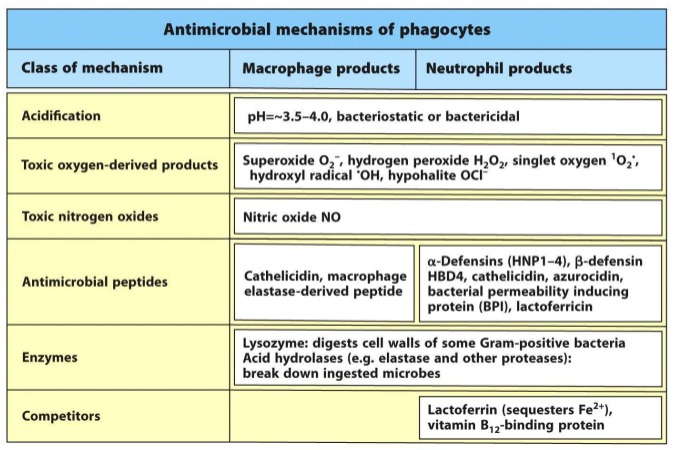
Acidification
• Enzymes
• Anti-microbial peptides
• Metabolic competitors
• Reactive Oxygen and Nitrogen Species (ROS, RNS)
• Enzymes
• Anti-microbial peptides
• Metabolic competitors
• Reactive Oxygen and Nitrogen Species (ROS, RNS)
19
New cards
What mediates phagocytosis?
“Eating” a target doesn’t happen by chance – you don’t just bump into your target
Phagocytosis is mediated by receptors on the phagocytes recognizing conserved residues on the target pathogen
The residues on the targets correspond to both pathogen derived molecules and host-derived molecules (opsonins)
Phagocytosis is mediated by receptors on the phagocytes recognizing conserved residues on the target pathogen
The residues on the targets correspond to both pathogen derived molecules and host-derived molecules (opsonins)
20
New cards
Define: Lysosome
Most of the digestive “punch” of phagocytosis is provided by the lysosome
Lysosomes are membrane bound vesicles located within the cytoplasm
They contain a diverse array of peptides, enzymes and other molecules
Lysosomes are membrane bound vesicles located within the cytoplasm
They contain a diverse array of peptides, enzymes and other molecules
21
New cards
How are lysosomes activated/regulated?
At normal physiological pH (~7) most of the enzymes are not active.
Mature lysosome is acidic (
Mature lysosome is acidic (
22
New cards
What are some contents of lysosomes?
- Acid Phosphatase
- Nucleases (DNAse, RNAse)
- Protein Digesting Enzymes
- Carbohydrate Digesting Enzymes
- Lipid Digesting Enzymes
- Defensins
- Nucleases (DNAse, RNAse)
- Protein Digesting Enzymes
- Carbohydrate Digesting Enzymes
- Lipid Digesting Enzymes
- Defensins
23
New cards
Describe the process of lysosome protein sorting.
1. Proteins targeted for the lysosome are “tagged” with a mannose-6-phosphate (M6P) residue
2. Binds to M6P receptor in the ER
3. M6P receptor containing vesicles bud off of the ER and fuse with late endosomes
4. These late endosomes begin to pump H+ ions in, lowering the pH of the vesicle
5. At low pH, the enzymes dissociate from the M6P receptor and as such become activated
6. M6P receptors are recycled back to the ER leaving the active enzymes within the mature lysosome
2. Binds to M6P receptor in the ER
3. M6P receptor containing vesicles bud off of the ER and fuse with late endosomes
4. These late endosomes begin to pump H+ ions in, lowering the pH of the vesicle
5. At low pH, the enzymes dissociate from the M6P receptor and as such become activated
6. M6P receptors are recycled back to the ER leaving the active enzymes within the mature lysosome
24
New cards
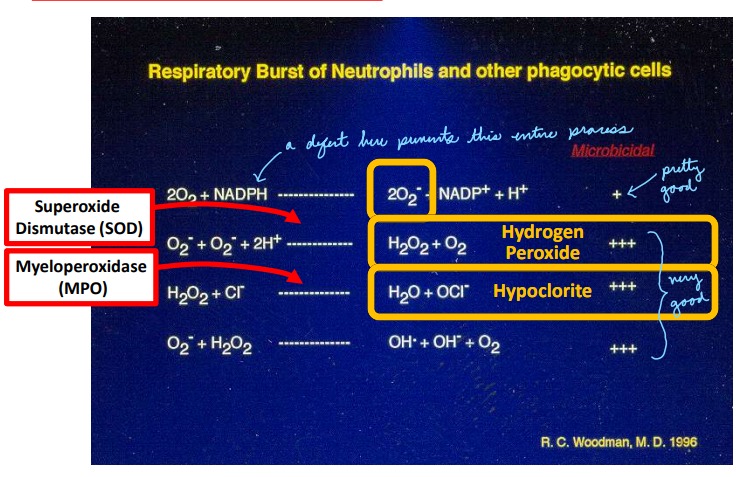
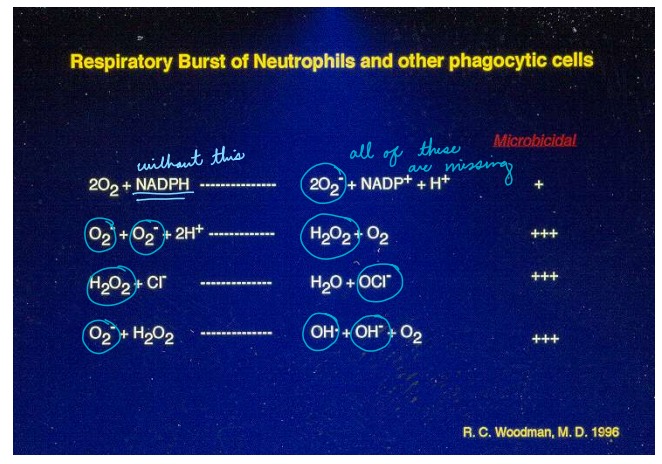
Describe: Oxidative Burst
Occurs mainly in phagosomes (especially neutrophils)
Phagocytes are able to use a series of enzymes to generate reactive oxygen and nitrogen species (ROS, RNS). For ROS – the entire system revolves around superoxide O2- , which forms hypochlorite (bleach) and hydrogen peroxide.
Phagocytes are able to use a series of enzymes to generate reactive oxygen and nitrogen species (ROS, RNS). For ROS – the entire system revolves around superoxide O2- , which forms hypochlorite (bleach) and hydrogen peroxide.
25
New cards
How does ROS kill?
• damage of DNA
• oxidations of polyunsaturated fatty acids in lipids (lipid peroxidation)
• oxidations of amino acids in proteins
• inactivate specific enzymes by oxidation of co-factors
• oxidations of polyunsaturated fatty acids in lipids (lipid peroxidation)
• oxidations of amino acids in proteins
• inactivate specific enzymes by oxidation of co-factors
26
New cards
Why doesn't ROS hurt us?
Due to membrane localization of NADPH oxidase components ROS are released directly into vesicles
ROS are EXTREMELY reactive and, as such, have ETREMELY SHORT half-lives (seconds or shorter)
ROS are EXTREMELY reactive and, as such, have ETREMELY SHORT half-lives (seconds or shorter)
27
New cards
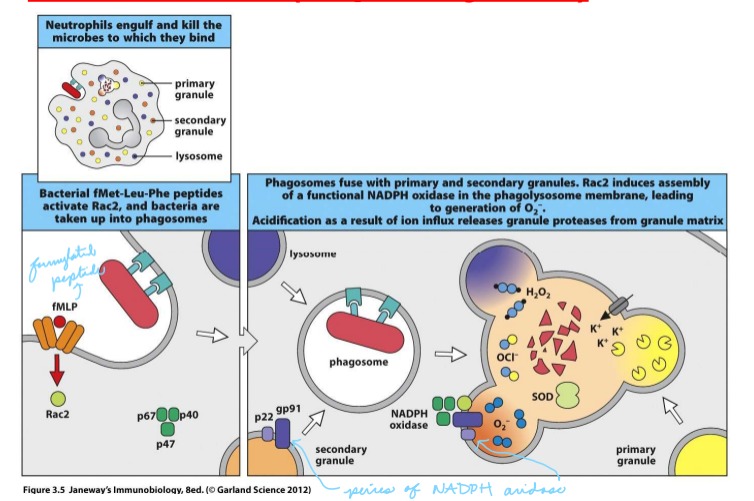
What is the mechanism that causes oxidative burst?
Following phagocytosis, the NADPH oxidase complex forms in the phagolysosome membrane
Some components of NADPH oxidase are located within the membrane of granules, others are cytoplasmic – all must
assemble to form a functional enzymatic complex
NADPH oxidase is responsible for the generation of superoxide
Some components of NADPH oxidase are located within the membrane of granules, others are cytoplasmic – all must
assemble to form a functional enzymatic complex
NADPH oxidase is responsible for the generation of superoxide
28
New cards
What is Chronic Granulomatous Disease (CGD)
Recurrent bouts of infection (pneumonia, abscesses)
Frequently fungal or catalase positive bacteria
Disease is heritable and is cause by a mutation in any one of a number of the subunits of NADPH oxidase
Diagnosis with the nitroblue-tetrazolium (NBT) test (turns blue when oxidized by super oxide, remains colourless if they have CGD)
Frequently fungal or catalase positive bacteria
Disease is heritable and is cause by a mutation in any one of a number of the subunits of NADPH oxidase
Diagnosis with the nitroblue-tetrazolium (NBT) test (turns blue when oxidized by super oxide, remains colourless if they have CGD)
29
New cards
What is a Neutrophil Extracellular Trap (NET) why do cells use them?
Neutrophils cannot “outrun” a mobile pathogen. Need a mechanism to “catch” pathogens flying by in the blood
Some cells can release the DNA contained in their nucleus and spread it around like a “net” to catch pathogens
Comprised of decondensed chromatin (nuclear DNA) covered in histones, and granular proteins (lysozyme, elastase, myeloperoxidase) Very cytotoxic – can kill pathogens – also causes host cell damage
Some cells can release the DNA contained in their nucleus and spread it around like a “net” to catch pathogens
Comprised of decondensed chromatin (nuclear DNA) covered in histones, and granular proteins (lysozyme, elastase, myeloperoxidase) Very cytotoxic – can kill pathogens – also causes host cell damage
30
New cards
What triggers NET production?
Neutrophils need to be first activated by PAMPS
Platelets then bind to activated neutrophils in the blood vessels and trigger NET production
Platelets then bind to activated neutrophils in the blood vessels and trigger NET production