Physics: Module 6 - NUCLEAR & PARTICLE PHYSICS
0.0(0)
0.0(0)
Card Sorting
1/56
Earn XP
Description and Tags
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
57 Terms
1
New cards
why had they initially proposed that the electron must exist within a nucleus
* it was the only other fundamental particle we knew about
* it had been observed in certain radioactive decays that electrons are emitted from the nucleus
* huge conflicts with this and Heisenberg Uncertainty Principle
* it had been observed in certain radioactive decays that electrons are emitted from the nucleus
* huge conflicts with this and Heisenberg Uncertainty Principle
2
New cards
strong nuclear force
* acts over a very small distance (about 3fm/3x10^-15m)
* independent of charge (acts on both protons and neutrons)
* stronger than the electromagnetic force in the nucleus (but on by about 2 order)
* can be repulsive and attractive (below 0.5fm = 0.5x10^-15m)
* independent of charge (acts on both protons and neutrons)
* stronger than the electromagnetic force in the nucleus (but on by about 2 order)
* can be repulsive and attractive (below 0.5fm = 0.5x10^-15m)
3
New cards
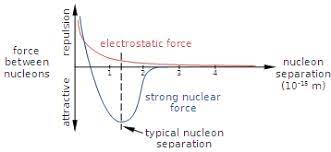
what does this graph show?
* resultant force on proton (e.g. the combines effect of electromagnetic and strong nuclear force)
* electric force dominates at large separations
* strong nuclear force dominates at small separations
* electric force dominates at large separations
* strong nuclear force dominates at small separations
4
New cards
why must there be more neutrons than protons in a large nuclei?
* outer protons become increasingly far apart
* strong nuclear force becomes less dominant
* need to increase as EM repulsion could cause them to fly apart
* add more neutrons to increase strong nuclear force without increasing EM repulsion
* strong nuclear force becomes less dominant
* need to increase as EM repulsion could cause them to fly apart
* add more neutrons to increase strong nuclear force without increasing EM repulsion
5
New cards
equation relating nuclear density to nuclear number
* V is prop to m, therefore V is prop to R^3, so R^3 is prop to m
* mass of nucleus is determined by m = AMp
* A = nuclear number, Mp = mass of proton
* note: mass of proton and neutron are the same
R^3 is prop. to AMp
R^3 = cAMp
* c = constant
R = (CAMp)^1/3
R = (CMp)^1/3 \* A^1/3
* (CMp)^1/3 is pre calculated as r(o) = 1.2x10^-15
__**R = r(o) * A^1/3**__
* where R = radius of nucleus, A = nucleus number
* mass of nucleus is determined by m = AMp
* A = nuclear number, Mp = mass of proton
* note: mass of proton and neutron are the same
R^3 is prop. to AMp
R^3 = cAMp
* c = constant
R = (CAMp)^1/3
R = (CMp)^1/3 \* A^1/3
* (CMp)^1/3 is pre calculated as r(o) = 1.2x10^-15
__**R = r(o) * A^1/3**__
* where R = radius of nucleus, A = nucleus number
6
New cards
matter and antimatter
* every fundamental particle that exists has an anti-particle
* these have the same rest mass but opposite charge
* these have the same rest mass but opposite charge
7
New cards
what happens if a particle meets its anti-particle?
they will annihilate to produce a pair of high energy photons
8
New cards
sub-atomic particles
* what are the two main families of particles?
* What are their characteristics?
* what are the two main families of particles?
* What are their characteristics?
Leptons:
* fundamental particles
* can’t be broken down further, not made up of different particles
Hadrons:
* any particle made up of quarks which feels the strong force
* has two groups:
* baryons (heavy) and mesons (middle)
* fundamental particles
* can’t be broken down further, not made up of different particles
Hadrons:
* any particle made up of quarks which feels the strong force
* has two groups:
* baryons (heavy) and mesons (middle)
9
New cards
Define the two groups of hadrons
**Baryons:**
Quarks can combine in ==triplets== (all quarks or antiquarks) to form a Baryon
**Mesons:**
Quarks can also combine in ==quark - antiquark pairs== to form a meson
* note: anything that is a baryon has a baryon number of 1
Quarks can combine in ==triplets== (all quarks or antiquarks) to form a Baryon
**Mesons:**
Quarks can also combine in ==quark - antiquark pairs== to form a meson
* note: anything that is a baryon has a baryon number of 1
10
New cards
state the baryon number, strangeness, and lepton number of fundamental particles (hadron only)
up (u)
* B# = 1/3
* S = 0
* L# = 0
anti-up (u with a line on top)
* B# = -1/3
* S = 0
* L# = 0
down (d)
* B# = 1/3
* S = 0
* L# = 0
anti-down (d with a line on top)
* B# = -1/3
* S = 0
* L# = 0
strange (s)
* B# = 1/3
* S = -1
* L# = 0
anti-strange (s with a line on top)
* B# = -1/3
* S = 1
* L# = 0
* B# = 1/3
* S = 0
* L# = 0
anti-up (u with a line on top)
* B# = -1/3
* S = 0
* L# = 0
down (d)
* B# = 1/3
* S = 0
* L# = 0
anti-down (d with a line on top)
* B# = -1/3
* S = 0
* L# = 0
strange (s)
* B# = 1/3
* S = -1
* L# = 0
anti-strange (s with a line on top)
* B# = -1/3
* S = 1
* L# = 0
11
New cards
state the baryon number, strangeness, and lepton number of fundamental particles (lepton only)
electron (e-)
* B# = 0
* S = 0
* L# = 1
positron (e+)
* B# = 0
* S = 0
* L# = -1
electron neutrino (curly Ve)
* B# = 0
* S = 0
* L# = 1
antielectron neutrino (curly Ve with a line on top)
* B# = 0
* S = 0
* L# = -1
* B# = 0
* S = 0
* L# = 1
positron (e+)
* B# = 0
* S = 0
* L# = -1
electron neutrino (curly Ve)
* B# = 0
* S = 0
* L# = 1
antielectron neutrino (curly Ve with a line on top)
* B# = 0
* S = 0
* L# = -1
12
New cards
* gravitational force acts on …
* electromagnetic force acts on …
* strong force acts on …
* weak force acts on …
* electromagnetic force acts on …
* strong force acts on …
* weak force acts on …
* anything with mass
* charged objects
* hadrons (quarks, baryons, and mesons) only
* hadrons and leptons
* charged objects
* hadrons (quarks, baryons, and mesons) only
* hadrons and leptons
13
New cards
force mediators:
* electromagnetic force is carried by …
* strong force is carried by …
* weak force is carried by …
* electromagnetic force is carried by …
* strong force is carried by …
* weak force is carried by …
* the photon
* the gluon
* the gauge bosons (W+, W-, Z^0)
* the gluon
* the gauge bosons (W+, W-, Z^0)
14
New cards
conservation laws
* in all interactions, the following must be conserved:
* in all interactions, the following must be conserved:
* mass-energy
* charge
* momentum
* spin
* baryon number
* lepton number
* strangeness
* charge
* momentum
* spin
* baryon number
* lepton number
* strangeness
15
New cards
what are weak interaction responsible for?
beta decay
16
New cards
what is the equation for beta-minus decay in terms of quarks
d → u + e- +Ve (curly V with a line on top)
or
d → u + beta- +Ve (curly V with a line on top)
* when a neutron becomes a proton, a down quark turns into an up quark
or
d → u + beta- +Ve (curly V with a line on top)
* when a neutron becomes a proton, a down quark turns into an up quark
17
New cards
what’s happening in beta-minus decay?
* the weak interaction causes a down quark to turn into an up quark by emitting W- boson
* this almost immediately decays into an electron and anti-electron neutrino (Ve - curly V with line on top)
* W- gauge boson emitted
* this almost immediately decays into an electron and anti-electron neutrino (Ve - curly V with line on top)
* W- gauge boson emitted
18
New cards
beta-minus decay equation
n → p+ + e- + Ve (curly V with line on top)
19
New cards
outside the nucleus, neutrons are … . They decay after …
unstable, about 15 minutes via this weak interaction
20
New cards
beta plus decay and beta plus decay in terms of quarks
* what is emitted?
* what is emitted?
p+ → n + e+ + Ve (curly V)
* u → d + e+ + Ve
OR
* u → d + beta+ + Ve
* W+ gauge boson is emitted
* up quark turns into a down quark
* electron neutrino is added due to conservation laws
* u → d + e+ + Ve
OR
* u → d + beta+ + Ve
* W+ gauge boson is emitted
* up quark turns into a down quark
* electron neutrino is added due to conservation laws
21
New cards
binding energy
the energy required to separate a nucleus into its constituent parts
22
New cards
why is there a difference between the mass of the separate particles and of the whole atom?
because work has to be done to separate the particles
23
New cards
what does a higher binding energy mean?
more stable
24
New cards
* light nuclei have … binding energy
* Fe- has the … binding energy per nucleon making it …
* for isotopes with A>20 there’s …
* He - 4 is an anomaly which is …
* Fe- has the … binding energy per nucleon making it …
* for isotopes with A>20 there’s …
* He - 4 is an anomaly which is …
* low
* greatest, the most stable nucleus
* little variation in binding energy
* unusually stable
* greatest, the most stable nucleus
* little variation in binding energy
* unusually stable
25
New cards
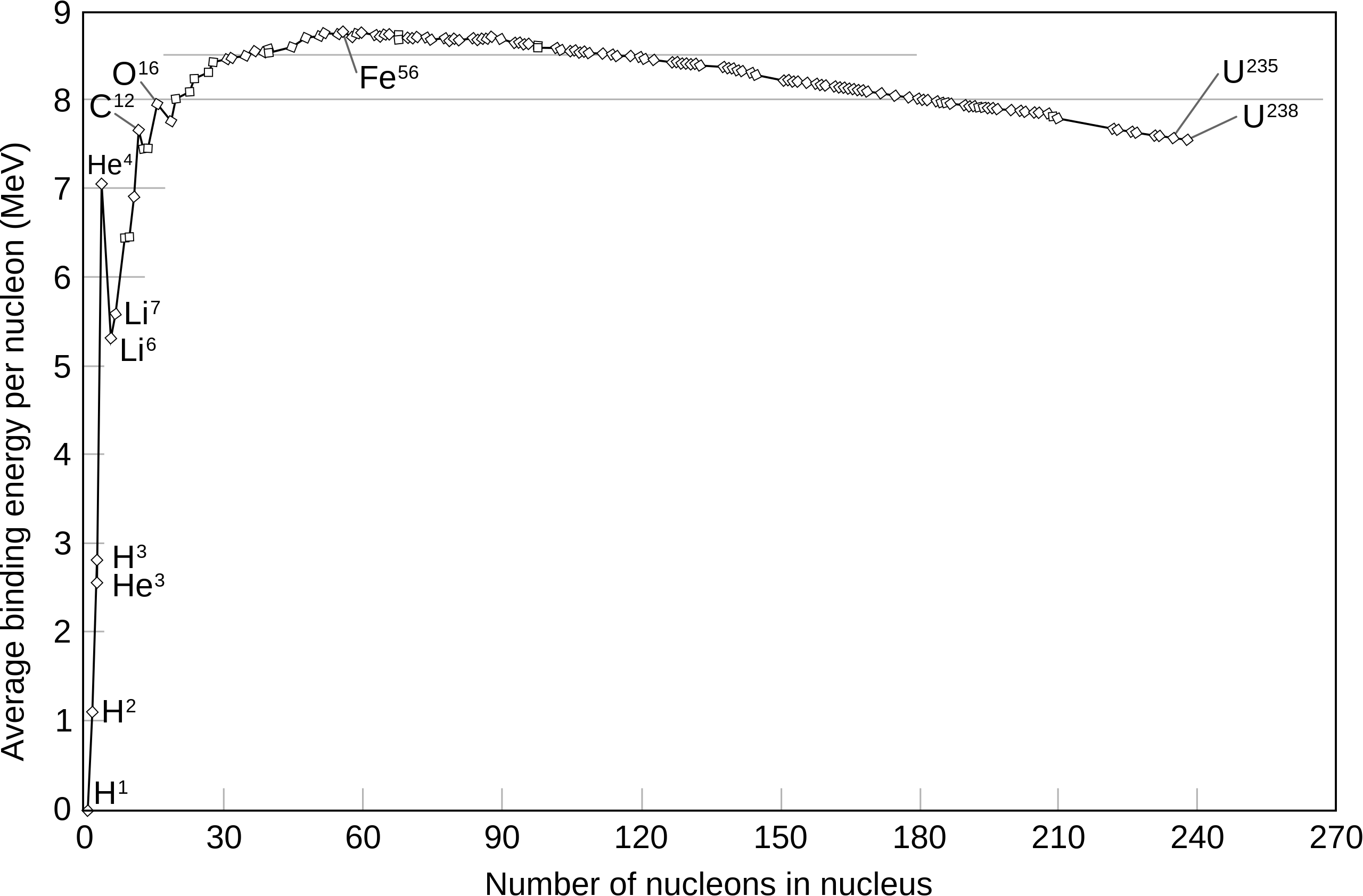
from which point is fusion and fission occurring?
the cutoff line is approx. at Fe
26
New cards
nuclear fusion
* for some lighter isotopes its energetically favourable to fuse together
* in these cases, the final particle will have less mass than the parent particles
* the final particle is more tightly bound than the parent particles and therefore more stable
* in these cases, the final particle will have less mass than the parent particles
* the final particle is more tightly bound than the parent particles and therefore more stable
27
New cards
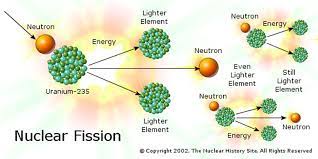
nuclear fission
for some heavy nuclei, it’s energetically favourable for them to split into lighter nuclei:
* a massive nucleus which is neutron-rich is unstable
* it’s held together by the strong force
* the nucleus distorts, if sufficiently distorted, the electrostatic repulsion between the protons may be strong enough to separate them
* greater distance from distortion
* two highly excited fission products are formed
* called daughter products
* the product nuclei become more stable by emitting neutrons
* a massive nucleus which is neutron-rich is unstable
* it’s held together by the strong force
* the nucleus distorts, if sufficiently distorted, the electrostatic repulsion between the protons may be strong enough to separate them
* greater distance from distortion
* two highly excited fission products are formed
* called daughter products
* the product nuclei become more stable by emitting neutrons
28
New cards
what is a thermal neutron?
slow neutron - roughly a few km/s
29
New cards
induced nuclear fission
* a thermal neutron is absorbed by the nucleus of a fissile atom (e.g. uranium-235)
* 2 to 5 high-speed neutrons are released - if slowed, these could go on to be absorbed by other nuclei causing further fission reactions - a chain reaction
* 2 to 5 high-speed neutrons are released - if slowed, these could go on to be absorbed by other nuclei causing further fission reactions - a chain reaction
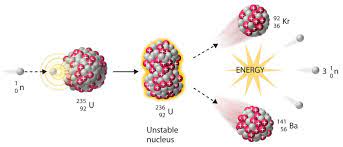
30
New cards
what are common nuclear fuels
uranium, plutonium, thorium
31
New cards
what is one of the best fissile material?
what is its half life?
what is its half life?
* uranium-235
* 710 million years
* only 0.7% of uranium is U-235
* 710 million years
* only 0.7% of uranium is U-235
32
New cards
what is the most abundant uranium isotope?
uranium-238
* half life of 4500 million years
* half life of 4500 million years
33
New cards
problems with a nuclear fission reactor
* the neutrons are travelling too quickly to be absorbed
* the neutrons are absorbed by U-238 nuclei
* some neutrons absorbed by materials in the reactor cause these materials to become radioactive
* the neutrons are absorbed by U-238 nuclei
* some neutrons absorbed by materials in the reactor cause these materials to become radioactive
34
New cards
control rods
* control the rate of fission within the reactor with rods of boron that can be raised or lowered between the fuel rods
* these control rods will absorb the neutrons and prevent further fission from being induced
* these control rods will absorb the neutrons and prevent further fission from being induced
35
New cards
moderator
* a material such as graphite or heavy water surrounds the individual fuel rods
* neutrons leaving the fuel rods undergo collisions with the atoms which acts to slow them down
* neutrons leaving the fuel rods undergo collisions with the atoms which acts to slow them down
36
New cards
nuclear fusion in the sun
* hydrogen nuclei (NOT ATOMS) fuse to produce Helium nuclei
* there’s electrostatic repulsion between nuclei and so they need very high temperatures (high velocities) to get close enough
* there must also be a very high density (and therefore pressure) to allow enough collisions to take place so that some do fuse
* there’s an overall decrease in mass which releases energy in the form of KE and photons
* there’s electrostatic repulsion between nuclei and so they need very high temperatures (high velocities) to get close enough
* there must also be a very high density (and therefore pressure) to allow enough collisions to take place so that some do fuse
* there’s an overall decrease in mass which releases energy in the form of KE and photons
37
New cards
advantages of fusion
* no radioactive waste products are directly formed
* almost unlimited supply of raw materials
* possible energy source for up to 1 million years
* almost unlimited supply of raw materials
* possible energy source for up to 1 million years
38
New cards
disadvantages of fusion
* need temperatures in excess of 100 million K
* at the moment we have to supply more energy that we get out
* at the moment we have to supply more energy that we get out
39
New cards
* basic equation of fusion
* where is deuterium found
* where is tritium found and what does it do
* where is deuterium found
* where is tritium found and what does it do
* tritium + deuterium → helium + neutron
* found naturally in seawater
* tritium is created
* lithium could surround the core
* this would absorb neutrons to become tritium
* lithium + neutron → tritium + helium
* found naturally in seawater
* tritium is created
* lithium could surround the core
* this would absorb neutrons to become tritium
* lithium + neutron → tritium + helium
40
New cards
radioactive decay
is spontaneous (unaffected by heat, pressure, pH, magnetic or electric fields) and is random (no way to predict when a certain nucleus will decay)
41
New cards
alpha emission
alpha: 2 neutrons, 2 protons
Pu → U + alpha + energy
* the numbers all add up!! conservation laws
* Pu - parent nucleus
* U - daughter nucleus (outcome)
* energy due to a decrease in mass - energy=mc^2
* Pu > U + alpha
Pu → U + alpha + energy
* the numbers all add up!! conservation laws
* Pu - parent nucleus
* U - daughter nucleus (outcome)
* energy due to a decrease in mass - energy=mc^2
* Pu > U + alpha

42
New cards
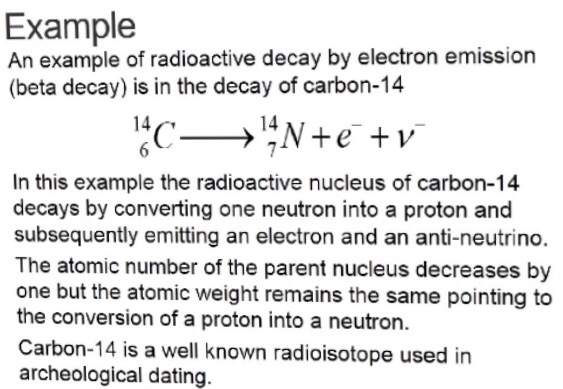
beta emission
* beta+ decay
* beta- decay
* beta+ decay
* beta- decay
beta: fast-moving electron
beta-
* C → N + beta + antielectron neutrino
* Ve- used to conserve lepton number conservation
beta+
* F → O + beta + energy + electron neutrino
beta-
* C → N + beta + antielectron neutrino
* Ve- used to conserve lepton number conservation
beta+
* F → O + beta + energy + electron neutrino
43
New cards
gamma emission
gamma: high energy electromagnetic wave (lander
44
New cards
when is gamma emitted
* as the nucleus settles into a lower energy state
* also emitted in alpha and beta decay
* also emitted in alpha and beta decay
45
New cards
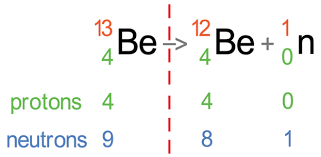
neutron emission
neutron is emitted from the nucleus
* Be → Be (isotope) + n
* Be → Be (isotope) + n
46
New cards
electron capture
proton-rich nuclide absorbs an electron from a low orbit, this turns a proton → neutron
* Al + e- → Mg + energy + electron neutrino
* Al + e- → Mg + energy + electron neutrino
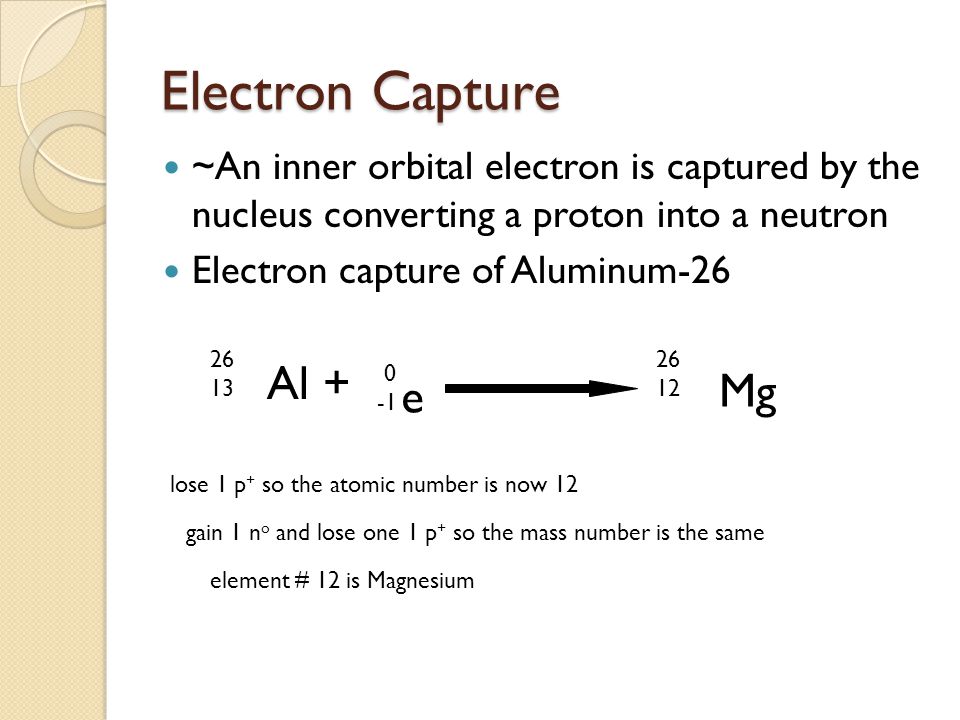
47
New cards
alpha, beta, gamma:
* charge
* how ionising
* how penetrating
* absorbed by
* change in parent nucleus
* mass
* typical speed of emission
* charge
* how ionising
* how penetrating
* absorbed by
* change in parent nucleus
* mass
* typical speed of emission
apha:
* +2, highly, weakly, paper/5cm air, loses 2 neutrons and 2 protons, 4.00151u, 1x10^6 m/s
beta:
* moderately, moderately, thin sheet of foil or 1m of air, 1 extra proton and 1 less neutron as neutron→proton, 0.00055u, 1x10^8 m/s
gamma:
* weakly, highly, 5cm lead, none, 0, c = 3x10^8 m/s
* +2, highly, weakly, paper/5cm air, loses 2 neutrons and 2 protons, 4.00151u, 1x10^6 m/s
beta:
* moderately, moderately, thin sheet of foil or 1m of air, 1 extra proton and 1 less neutron as neutron→proton, 0.00055u, 1x10^8 m/s
gamma:
* weakly, highly, 5cm lead, none, 0, c = 3x10^8 m/s
48
New cards
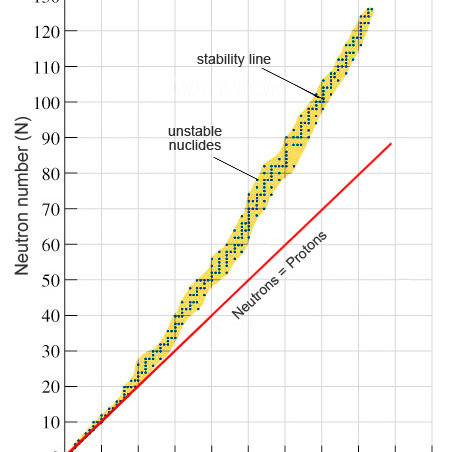
what does it mean if the value is…
* above the line
* below the line
what is the curve called?
when does alpha emission occur?
as you get a larger nucleus …
* above the line
* below the line
what is the curve called?
when does alpha emission occur?
as you get a larger nucleus …
* above the line - too many neutrons to be stable: beta- emission (neutron → proton)
* below the line - too many protons to be stable: beta+ emission (proton → neutron)
\
N-Z stability curve (N=#neutrons , z=#protons)
\
if Z>82 - too many protons and neutrons: alpha emission
\
the proportion of neutrons increase: repulsive electrostatic force increases therefore you require more strong force
* below the line - too many protons to be stable: beta+ emission (proton → neutron)
\
N-Z stability curve (N=#neutrons , z=#protons)
\
if Z>82 - too many protons and neutrons: alpha emission
\
the proportion of neutrons increase: repulsive electrostatic force increases therefore you require more strong force
49
New cards
activity (A)
the rate at which nuclei in a source decay and emit radioactive particles
* measured in Becquerels (Bq)
* 1 Bq = 1 decay/second
* measured in Becquerels (Bq)
* 1 Bq = 1 decay/second
50
New cards
count rate
number of detected particles per second
51
New cards
main equations in radioactive deacy
* A = lander\*N
* N = No\*e^(-lander\*t)
* A = Ao\*e^(-lander\*t)
* lander\*t1/2 = ln2 (t1/2 = half life)
* N = No\*e^(-lander\*t)
* A = Ao\*e^(-lander\*t)
* lander\*t1/2 = ln2 (t1/2 = half life)
52
New cards
decay constant
* the probability of radioactive decay of a nucleus per unit time
* units: s^-1 when activity is in Bq
* units: s^-1 when activity is in Bq
53
New cards
how to check if its exponential decay?
see if the half life remains constant from the graph (halves in equal times)
54
New cards
half life
* the mean time taken for the activity of the source to decrease by one half
* also the mean time taken for the # of radioactive nuclei to decrease by one half
* also the mean time taken for the # of radioactive nuclei to decrease by one half
55
New cards
* where is carbon-14 made?
* how does it decay?
* radiocarbon dating process?
* how does it decay?
* radiocarbon dating process?
* in the upper atmosphere by neutron capture
* decays elsewhere at the same rate - so there’s approximately a fixed quantity in the atmosphere
* living organisms take in the radioactive C-14 in the CO2 (plants) and glucose (animals) they absorb
* when the organism dies, they stop taking in the CO2 and so the amount of C-14 inside them starts decreasing as it radioactively decays (beta emission) into N-14
* its half-life is 5700 years
* decays elsewhere at the same rate - so there’s approximately a fixed quantity in the atmosphere
* living organisms take in the radioactive C-14 in the CO2 (plants) and glucose (animals) they absorb
* when the organism dies, they stop taking in the CO2 and so the amount of C-14 inside them starts decreasing as it radioactively decays (beta emission) into N-14
* its half-life is 5700 years
56
New cards
why can’t carbon dating be used on artefacts older than 100,000 years?
the activity would be so low that it could not be differentiated from the background
* proportion of C-14 to C-12 nuclei in dead and living objects and by comparing them it can be dated using N=No\*e^(-lander\*t)
* proportion of C-14 to C-12 nuclei in dead and living objects and by comparing them it can be dated using N=No\*e^(-lander\*t)
57
New cards
limitations of carbon dating
assumes the ratio of C-14 atoms to C-12 atoms has remained constant
* increased emission of CO2 may have reduced this ratio as would volcanic eruptions
* solar flares and the testing of nuclear weapons may also affect the ratio
* increased emission of CO2 may have reduced this ratio as would volcanic eruptions
* solar flares and the testing of nuclear weapons may also affect the ratio