Chapter 8: Energy Storage (Quiz)
1/39
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
40 Terms
Which type of widely used battery is not rechargeable?
Zinc-manganese alkaline
What condition must be met for a battery to be rechargeable?
the electrochemical reaction of the battery must be reversible
During the chemical reaction in an electrochemical cell,
oxidation occurs at the anode.
Which type of battery is widely used to store the excess energy generated by windmill farms and solar panels?
Lead-acid
Which is not a component of a fuel cell?
A timer
How does the reaction of hydrogen and oxygen to produce energy in a fuel cell differ from their interaction during the direct combustion of hydrogen and oxygen?
I, II, and III only
For safety and other practical reasons, the most logical use for hydrogen as a fuel in the near future is
in a fuel cell.
In a fuel cell,
reduction takes place at the cathode
What is the anode in a typical Li-ion battery composed of?
Graphite
Batteries are still preferred over supercapacitors for portable electronic applications. What factor(s) give rise to this preference?
Supercapacitors have low energy densities
Whenever a substance is oxidized,
some other substance must be reduced
A NiCd battery uses nickel and cadmium to produce a potential difference. Using these equations, answer the below question.
I. 2NiO(OH)(s) + 2H2O(l) + 2e− → 2Ni(OH)2(s) + 2OH−(aq)
II. Cd(s) + 2OH−(aq) → Cd(OH)2(s) + 2e−
III. Cd(s) + 2NiO(OH)(s) + 2H2O(l) → 2Ni(OH)2(s) + Cd(OH)2(s)
Which equation represents what takes place at the anode?
II
As fuel cells become more widely accepted and are used more, we will
be able to generate electricity in places where we cannot now do so.
What moves in the salt bridge from the cathode to the anode?
Anions
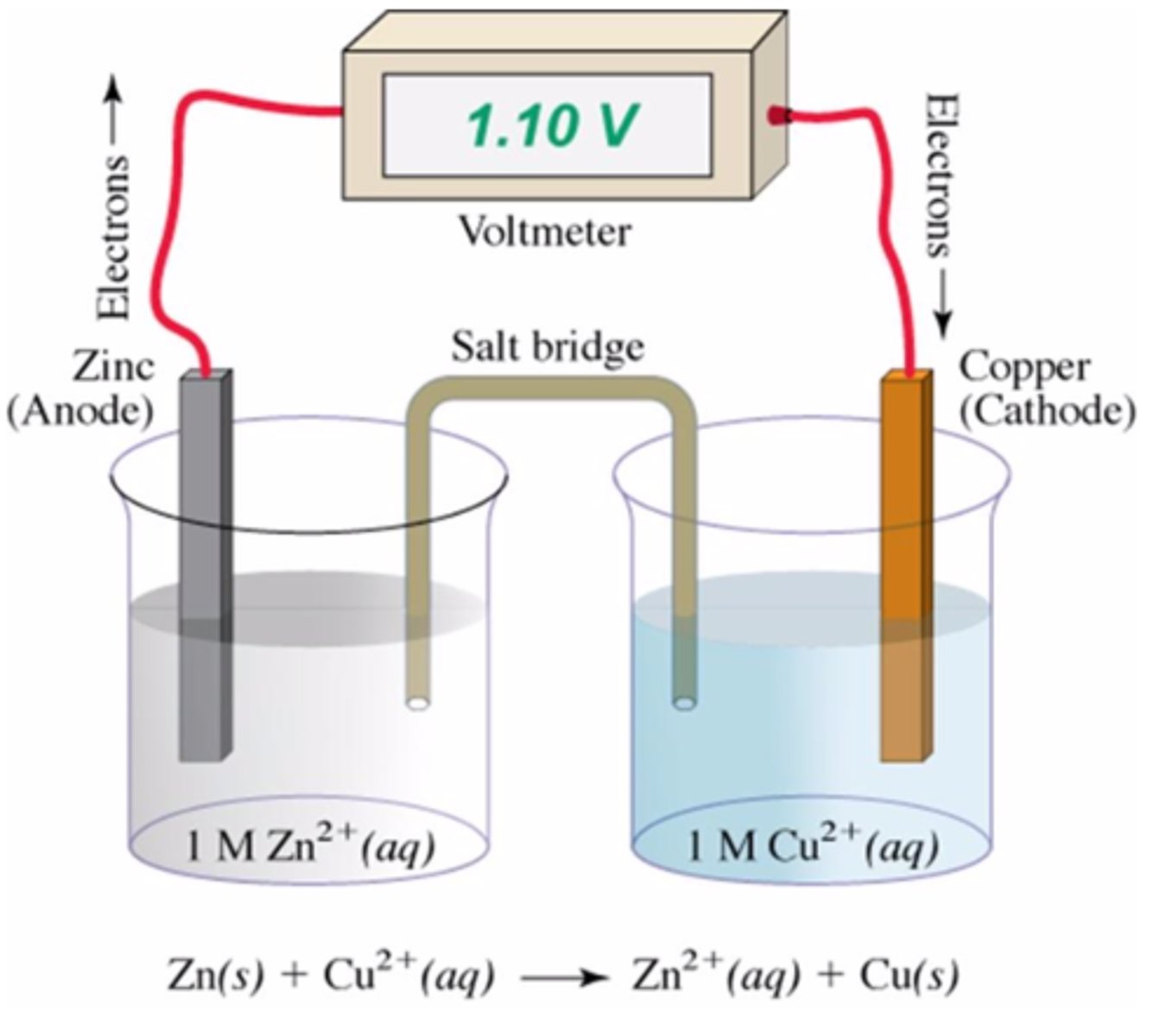
Which is not a necessary consideration for a battery designed to run a cell phone or portable MP3 player?
It must use liquid, aqueous solutions
In this electrochemical cell, the reduction half-reaction is
Cu2+(aq) + 2e− → Cu(s)
In an electrochemical cell, the anode is
the electrode at which oxidation takes place.
Consider Ohm's Law. Which of the following are correct?
Voltage is proportional to current
Which of the following batteries has the lowest volumetric and gravimetric energy densities?
Lead-acid
How do the interactions that are broken in water when it is boiled compare with those broken when water is electrolyzed?
Boiling water breaks intermolecular attractions and electrolysis breaks covalent bonds.
Which is not an advantage of using a solid electrolyte in a fuel cell?
It eliminates the need for an oxidant; only a fuel is required
Which of the following automotive energy storage devices would give rise to the fastest acceleration from zero to 60 mph?
Supercapacitors
Which type of battery is best for use in heart (cardiac) pacemakers?
Lithium-iodine
Which of the following statements is not true about PEM fuel cells?
PEM fuel cells rely on inexpensive catalysts
The opposite of a galvanic cell is
an electrolytic cell.
Which is the cathode in this galvanic cell?
The solid silver electrode
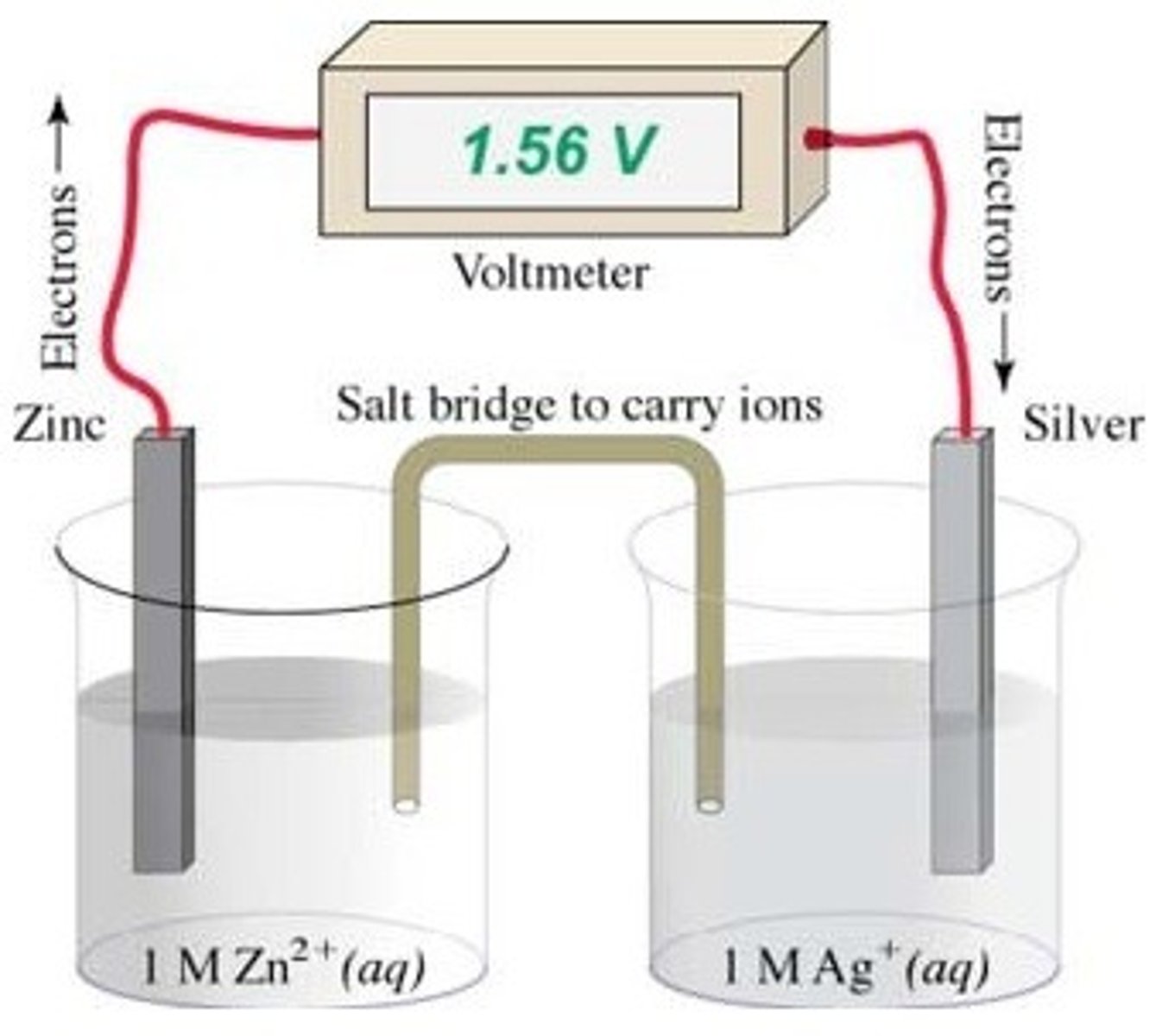
A fuel cell does not run down like a standard battery because
the reactants in a battery must be stored inside the battery, whereas the reactants for a fuel cell flow in as needed.
Why doesn't water naturally become hydrogen and oxygen gas when exposed to light?
Water doesn't absorb the proper wavelength of light to split
What is the primary determinant of the voltage developed by a battery?
The nature of the materials in the reaction
In general, a modern hybrid vehicle is less polluting than a standard vehicle because it runs on both a
gasoline engine and an electric motor run by a rechargeable battery
A NiCd battery uses nickel and cadmium to produce a potential difference. Using these equations, answer the below question.
I. 2NiO(OH)(s) + 2H2O(l) + 2e− → 2Ni(OH)2(s) + 2OH−(aq)
II. Cd(s) + 2OH−(aq) → Cd(OH)2(s) + 2e−
III. Cd(s) + 2NiO(OH)(s) + 2H2O(l) → 2Ni(OH)2(s) + Cd(OH)2(s)
Which equation represents the whole chemical reaction within the galvanic cell?
III
Which has not been suggested as a reasonably practical way to store large amounts of hydrogen in relatively small spaces for its use as a fuel?
Liquefy hydrogen under pressure and store it much as we do with liquefied natural gas today
Which of the following next-generation batteries would be good choices based on the future availability of its elements?
Al-ion
What energy change is associated with the reaction to obtain one mole of H2 from one mole of water vapor? The balanced equation is 2 H2O(g) → 2H2(g) + O2(g) and the relevant bond energies are: H — H = 436 kJ/mol; H — O = 467 kJ/mol; O — O = 146 kJ/mol; O - O = 498 kJ/mol.
+249 kJ
For the compound Fe3O4, what is the oxidation state of Fe?
Mixture between 2+ and 3+
The current through a wire is most closely related to the
Rate of electron flow through a wire
The aluminum-air battery is being considered for use in automobiles. In this battery, aluminum metal undergoes oxidation to Al3+ ions and forms Al(OH)3. O2 from the air undergoes reduction to OH− ions. Which half-reaction occurs at the anode?
Formation of Al3+ ions from aluminum
For the compound FeCl3, what is the oxidation state of Fe?
3+
A major advantage of a fuel cell over a standard battery is that
As long as oxygen and fuel are supplied, a fuel cell will not run down like a battery will
A NiCd battery uses nickel and cadmium to produce a potential difference. Using these equations, answer the below question.
I. 2NiO(OH) (s) + 2H2O(l) + 2e− → 2Ni(OH)2(s) + 2OH−(aq)
II. Cd(s) + 2OH−(aq) → Cd(OH)2 (s) + 2e−
III. Cd(s) + 2NiO(OH)(s) + 2H2O(l) → 2Ni(OH)2(s) + Cd(OH)2(s)
Which equation best represents the reduction half-reaction?
I