OIA1012 ALKANE & CYCLOALKANE
1/14
Earn XP
Description and Tags
Study of Hydrocarbons: Alkanes & Cycloalkane *Feel free to browse other sources on the profile :)
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
15 Terms
What are alkanes?
Saturated hydrocarbons with the formula CnH2n+2.
What is a homologous series?
A series of organic compounds with a similar general formula (CnH2n+2), similar chemical properties and gradation in physical properties due to same functional group.
Define structural isomerism in alkanes.
Isomers with the same formula but different connectivity of carbon atoms.
How does branching affect boiling points?
Increased branching lowers boiling points as it reduces surface area, resulting in weaker van der Waals forces.
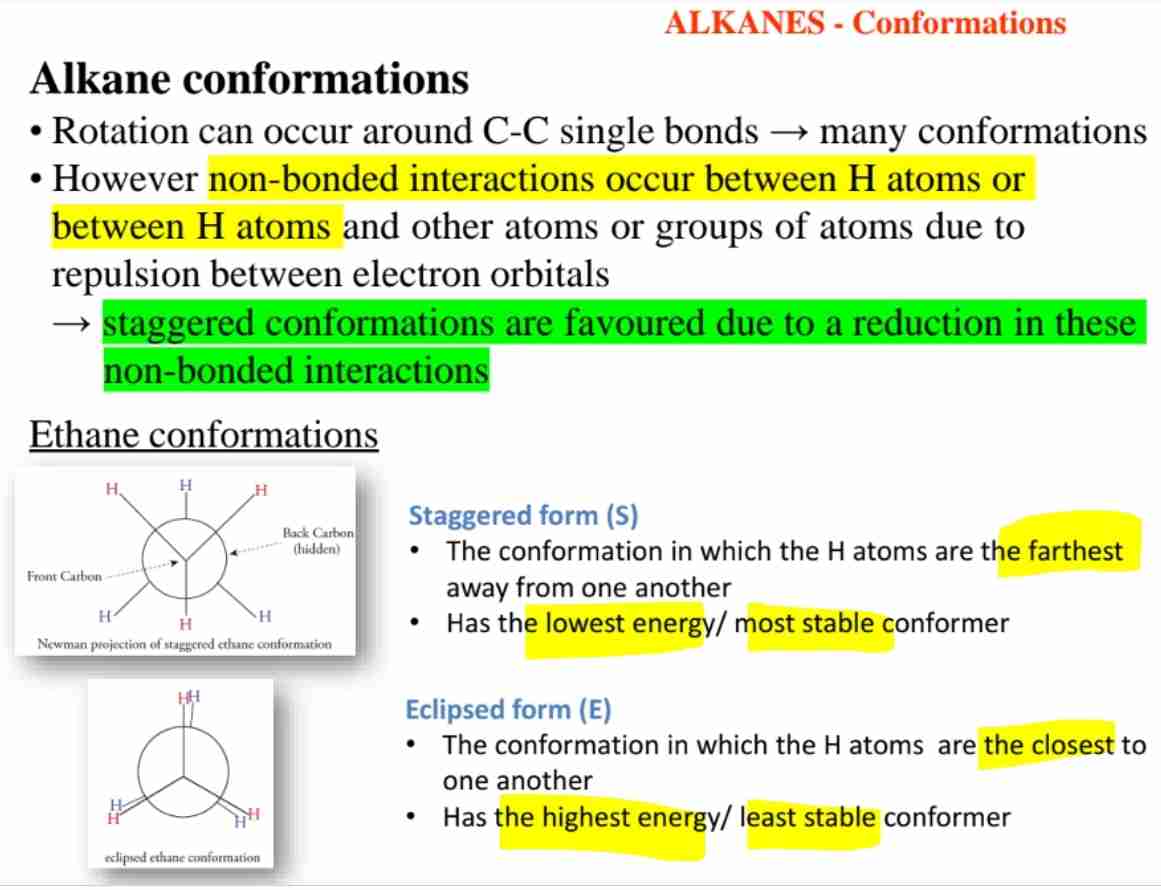
What are the conformations of ethane?
Staggered (most stable) and eclipsed (least stable).
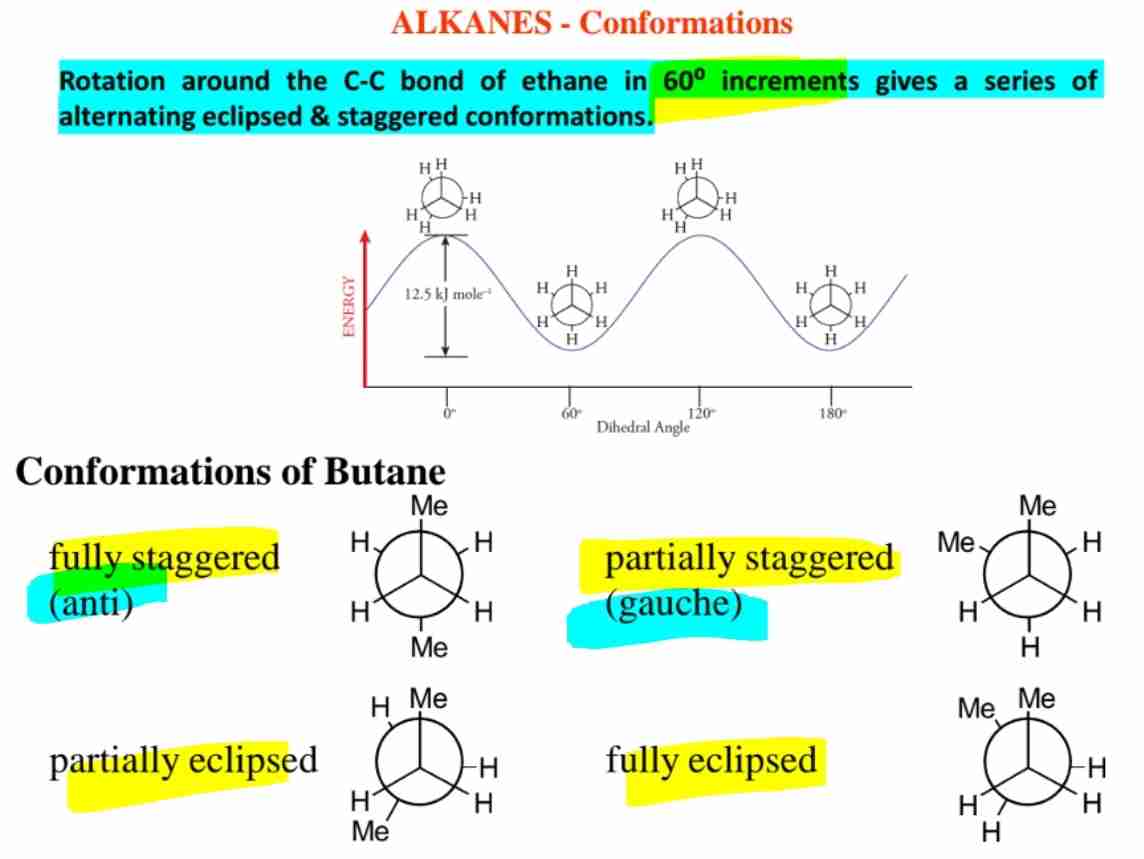
Define anti and gauche conformations in butane.
Anti: Methyl groups 180° apart (most stable). ;Gauche: Methyl groups 60° apart.
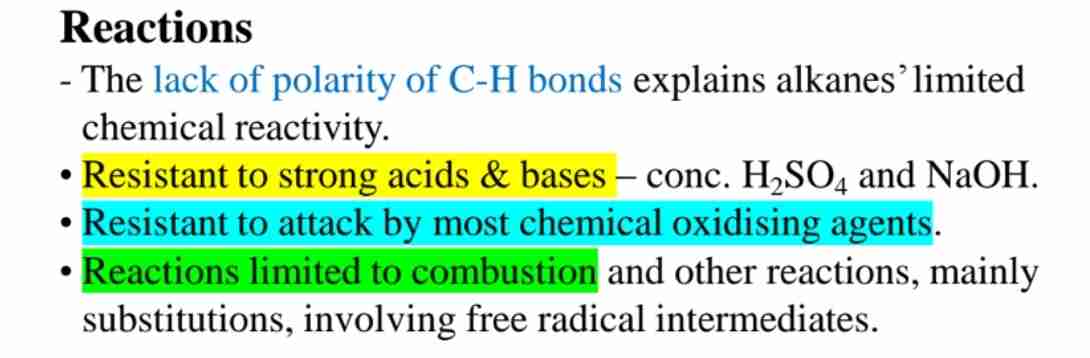
Why are alkanes chemically inert?
Due to non-polar C-H bonds and lack of functional groups.
What is the general formula of cycloalkanes?
CnH2n, indicating cyclic saturated hydrocarbons.
What is ring strain in cycloalkanes?
Strain due to angle distortion (Baeyer strain) and eclipsing interactions.
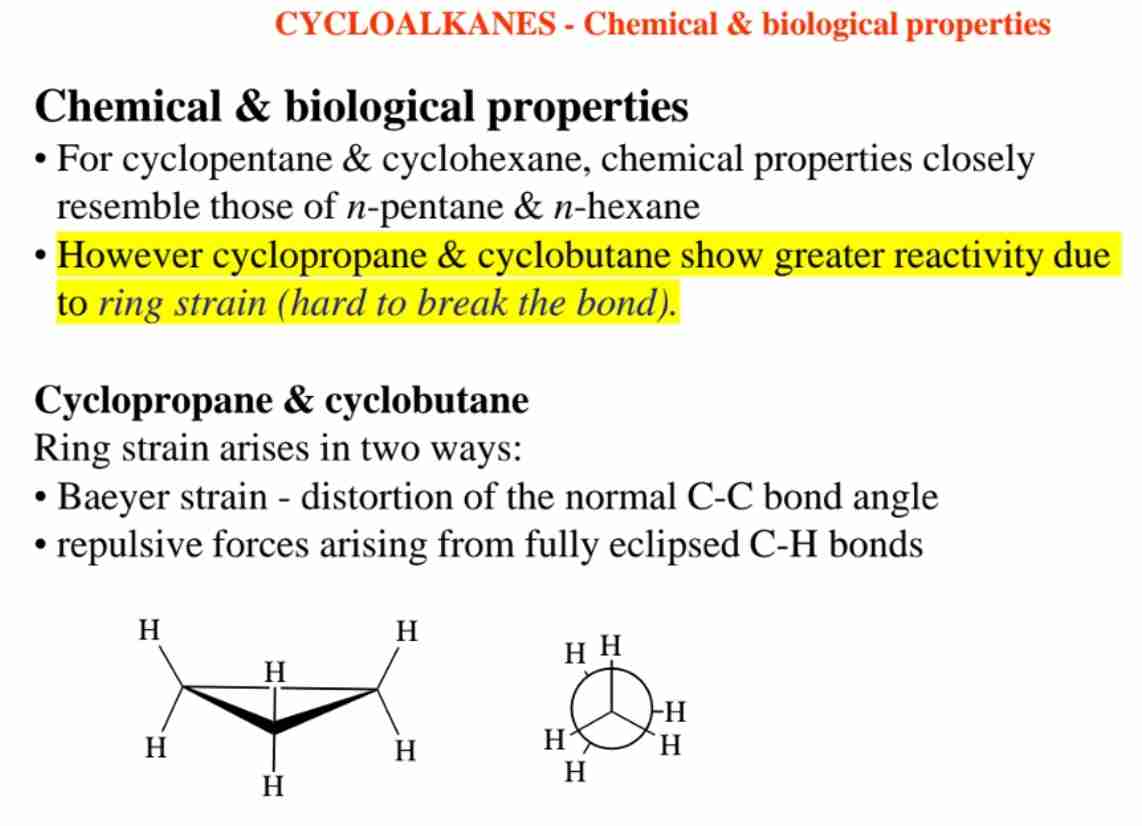
Which cycloalkane conformation is most stable?
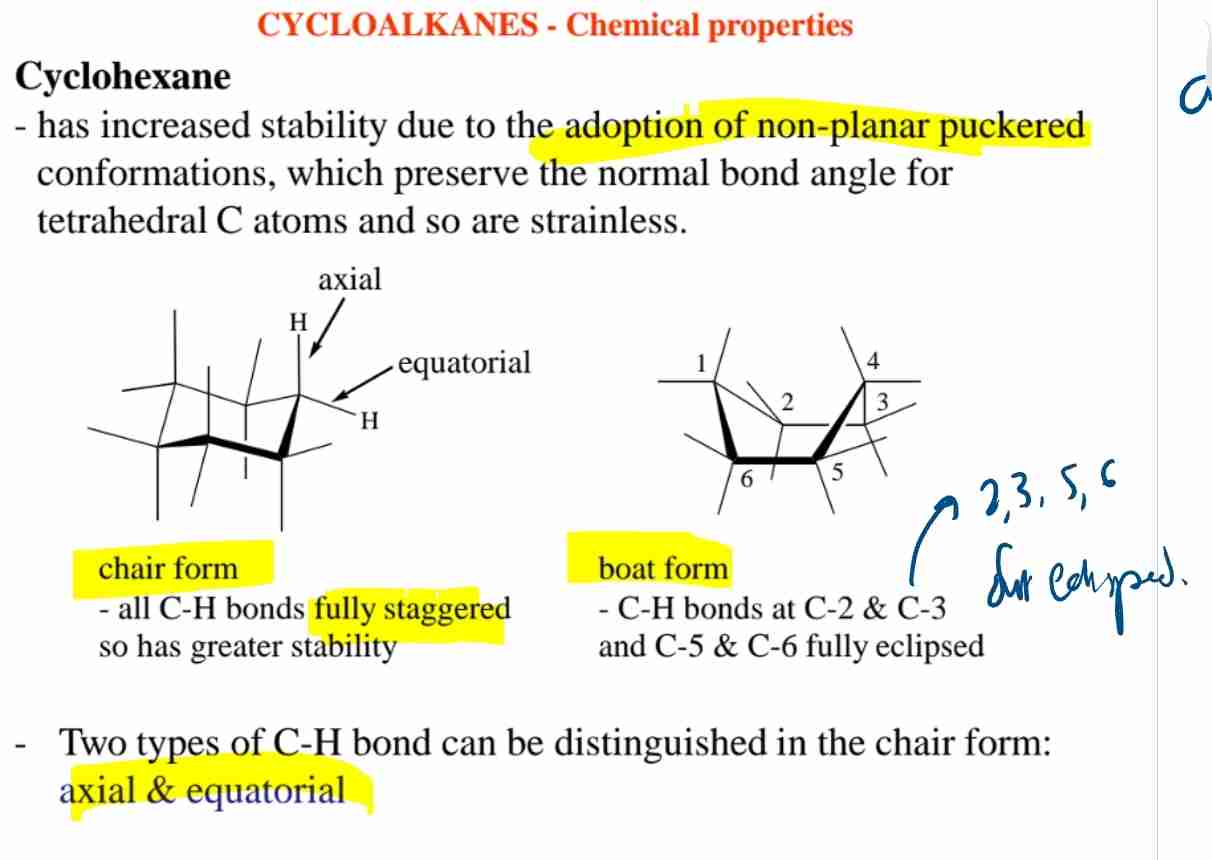
Chair form of cyclohexane due to minimized strain.
What is ring-flipping in cyclohexane?
The interconversion of chair forms, swapping axial and equatorial positions.
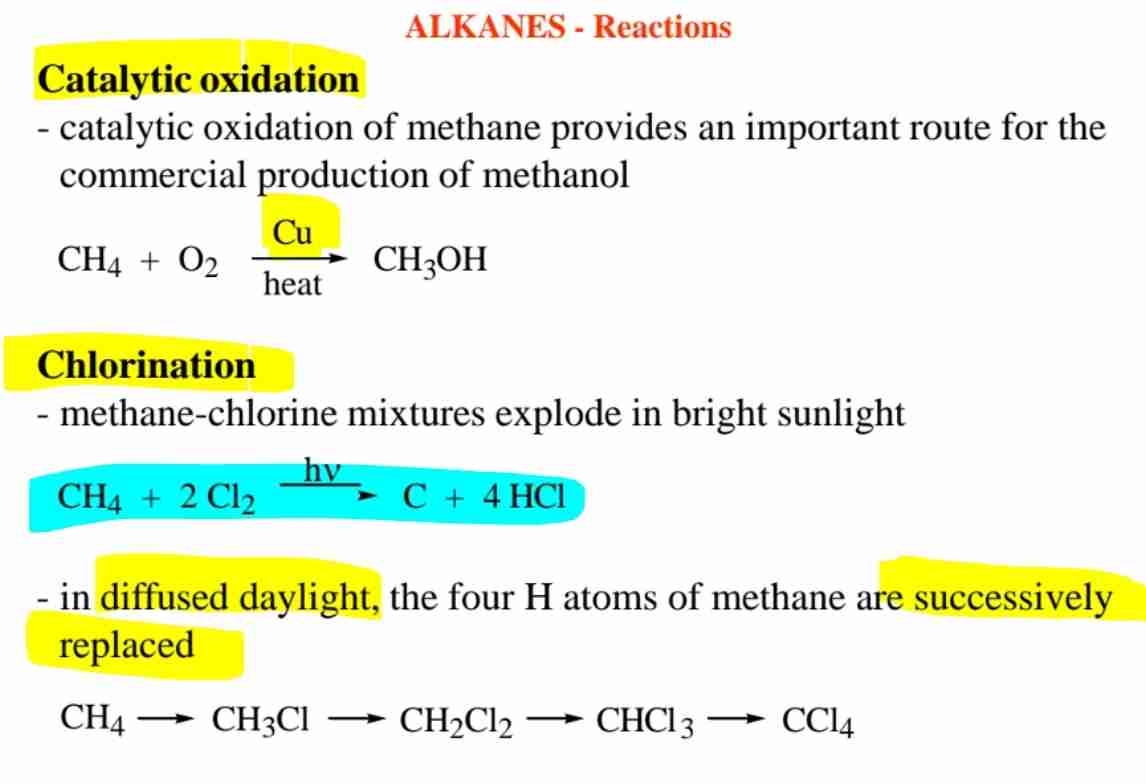
What are the reactions of alkanes?
Combustion, substitution (e.g., chlorination), and catalytic cracking.
What are the medicinal uses of alkanes?
Liquid paraffin as a laxative (orally); white petrolatum as an ointment base; hard paraffin as coat granules (delay drug absorption)
Why is cyclopropane used as an anesthetic?
Its high lipid solubility allows effective action in the nervous system.
What happens in the chlorination of methane?
Successive replacement of H atoms by Cl through free-radical mechanisms.