Unit 5: Energetics
1/10
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
11 Terms
1.1.0:
What can be deduced from the temperature change that accompanies chemical or physical change? (Solid ⇋ Liquid ⇋ Gas)
solid → liquid = endothermic
Liquid → gas = endothermic
All phase changes are accompanied by changes in the energy of a system. Changes from a more-ordered state to a less-ordered state (such as a liquid to a gas) are endothermic. Changes from a less-ordered state to a more-ordered state (such as a liquid to a solid) are always exothermic.
1.1.1:
What do chemical reactions involve?
Describe the difference between heat and temperature/What is the relationship between temperature and kinetic energy of particles?
Chemical reactions involve a transfer of energy between the system and the surroundings, while total energy is conserved.
Temperature is a measurement of the average kinetic energy of particles in an object. Heat is a flow of energy from an object at a higher temperature to an object at a lower temperature.
1.1.2:
What can reactions be described as (heat loss)?
Understand the temperature change (decrease or increase) that accompanies endothermic and exothermic reactions, respectively. What observations would you expect to make during an endothermic and an exothermic reaction?
Reactions are described as endothermic or exothermic, depending on the direction of energy transfer between the system and the surroundings.
Exothermic Reaction: Heat is transferred from system to surroundings, ΔH is negative. Endothermic Reaction: Heat is absorbed from surroundings, ΔH is positive.
1.1.3:
What does the relative stability of reactants or products indicate?
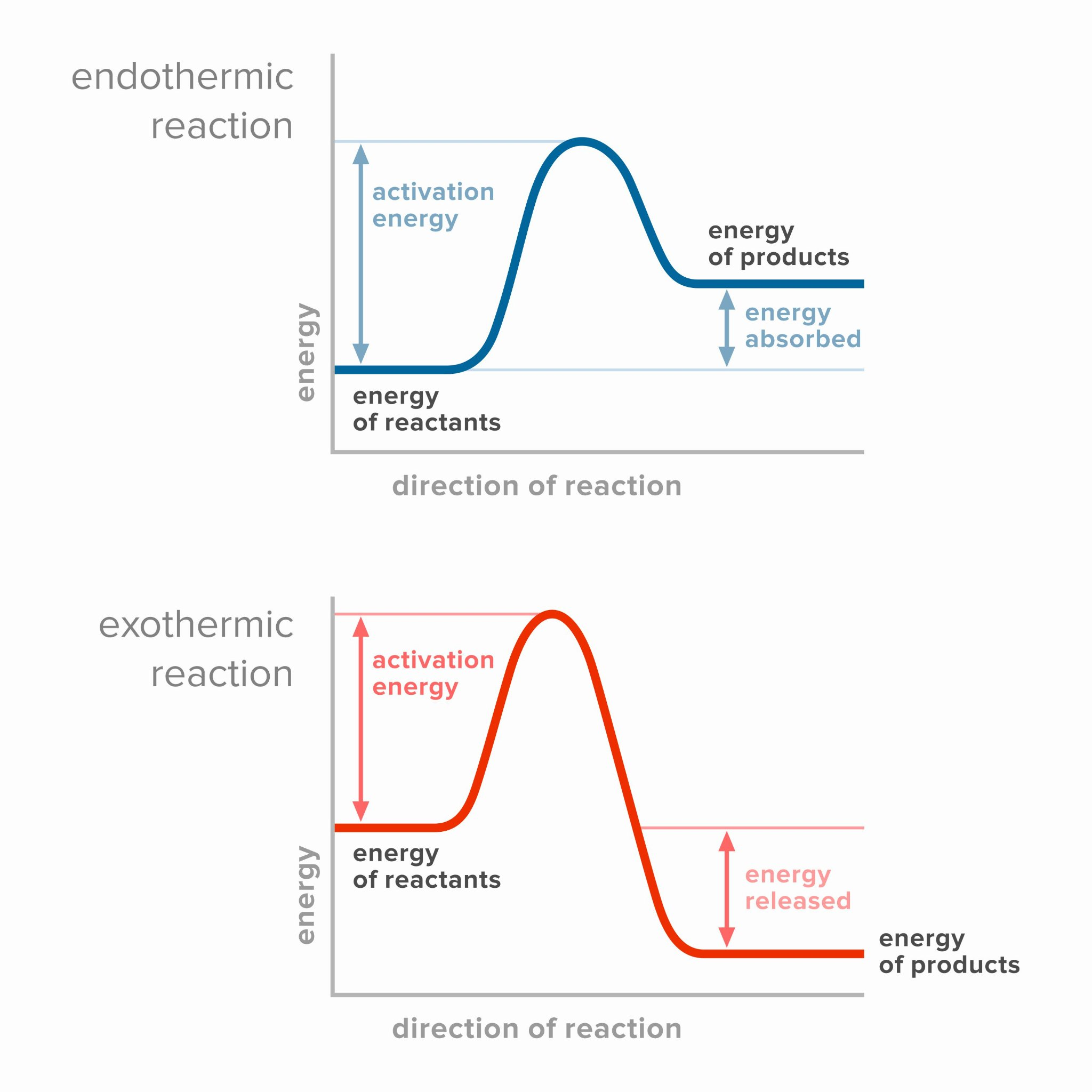
Sketch and interpret energy profiles for endothermic and exothermic reactions.
Most combustion reactions are exothermic; how does the bonding in N2 explain the fact that its combustion is endothermic?
The relative stability of reactants and products determines whether reactions are endothermic or exothermic.
For graph: Axes for energy profiles should be labeled as reaction coordinate (x), potential energy (y).
The endothermic combustion of N2 is due to the high energy required to break the strong triple bond between nitrogen atoms, leading to heat absorption in the process.
1.1.4:
What does standard enthalpy of change refer to and what is it’s symbol?
What are the units and equations for enthalpy change of a reaction (specific heat capacity)?
How can the enthalpy change for combustion reactions, such as for alcohols or food, be investigated experimentally?
Why do calorimetry experiments typically measure a smaller change in temperature than is expected from theoretical values?
The standard enthalpy change for a chemical reaction, ΔH⦵, refers to the heat transferred at constant pressure under standard conditions and states. It can be determined from the change in temperature of a pure substance.
The units of ΔH⦵ are kJ mol–1. The equation Q = mcΔT and the value of c, the specific heat capacity of water, are given in the data booklet.
The equations are Q = mcΔT and ΔH = −Q/n. Practice applying the equations in the calculation of the enthalpy change of a reaction.
Calorimetry, Spirit burner calorimetry, Enthalpy of neutralization & Thermometric titration as well as applying cooling curves data.
Smaller change measured arises from heat loss/gain to the surroundings, leading to lower observed temperature changes than predicted based on theoretical calculations.
1.2.0:
How does application of the law of conservation of energy help us to predict energy changes during reactions? What enthalpies do energy cycles apply?
Any reaction that produces a new substance must have the same overall energy change regardless of the route taken.
Energy cycles can apply enthalpy combustion, ΔHc⦵, and formation, ΔHf⦵, data.
Hess' law tells us that any difference in energy converting between two substances is independent of the route taken for the conversion
1.2.1:
Distinguish bond-breaking from bond-forming, in terms of their respective energy releases.
Calculate the enthalpy change of a reaction from given average bond enthalpy data.
Ex1: Find the average bond enthalpy of:
C3H8 + 5O2 → 3CO2 + 4H2O
Include explanation of why bond enthalpy data are average values and may differ from those measured experimentally.
Recall where average bond enthalpy values can be found in the data booklet:
______________________________
How would you expect bond enthalpy data to relate to bond length and polarity?
How does the strength of a carbon– halogen bond affect the rate of a nucleophilic substitution reaction? (NOT ON TEST)
Bond-breaking absorbs and bond-forming releases energy.
Answer for Ex1: -2034 Kjmol-1 (answer found on MSJ Chem “Calculating ΔH using average bond enthalpies”)
Values of enthalpy calcuated using the bond enthalpy terms are often far from the values found experimentally. This is due to the fact that they are average values and do not take into account situations in which the bond strength is different from the average
Average bond enthalpy values are given in TABLE 11 of the data booklet.
__________________________
Increased bond length = low polarity and weaker bond. Stronger polarity results in stronger and shorter bonds due to increased electrostatic attraction.
The rate of nucleophilic substitution reactions in halogenoalkanes is inversely proportional to the bond strength of the carbon-halogen bond. In bromoethane, the carbon-bromine (C-Br) bond is weaker than the carbon-chlorine (C-Cl) bond in chloroethane.
1.2.2:
State Hess’s law and apply the law to calculate enthalpy changes in multistep reactions.
Hess’s law states that the enthalpy change for a reaction is independent of the pathway between the initial and final states.
1.2.3:
Define Standard enthalpy changes of combustion, ΔHc⦵, and formation, ΔHf⦵ and state what equations they are used for,
Deduce equations and solutions to problems involving these terms.
where may the enthalpies of combustion and formation be found in the data booklet?
Would you expect allotropes of an element, such as diamond and graphite, to have different ΔHf⦵ values?
Standard enthalpy changes of combustion, ΔHc⦵, and formation, ΔHf⦵, data are used in thermodynamic calculations.
The enthalpy of formation is the energy change when one mole of a substance is made from its constituent elements in their standard states.
The enthalpy of combustion of a substance is defined as the heat energy given out when one mole of a substance burns completely in oxygen.
Enthalpies of combustion are found on table 13, and enthalpies of formation are found on table 12 of the data booklet.
Different allotropes have different arrangements of atoms, leading to different ΔHf⦵ values. Important: The most stable allotrope under standard conditions is assigned a ΔHf⦵ of zero.
1.2.4:
An application of Hess’s law uses enthalpies of _____ data or enthalpy of ______ data to calculate the ________ of a reaction.
What equations can be used to calculate enthalpy changes of a reaction using ΔH f⦵ data or ΔHc⦵ data?
Which page on the data booklet contains the equations to determine ΔHf⦵ data or ΔHc⦵ data?
An application of Hess’s law uses enthalpy of formation data or enthalpy of combustion data to calculate the enthalpy change of a reaction.
ΔH⦵ = Σ (ΔHf⦵ products) − Σ (ΔHf⦵ reactants)
ΔH⦵ = Σ (ΔHc⦵ reactants) − Σ (ΔHc⦵ products)
Not sure what page it’s referring to, but ΔHf⦵ can be found on table 12, ΔHc⦵ on table 13.
1.2.5:
Define a Born-Haber cycle.
What enthalpy changes does a born haber cycle include, and where can they be found in the data booklet?
Interpret and determine values from a Born–Haber cycle for compounds composed of univalent and divalent ions (±1 or ±2 charge/binding sites):
Ex1: Given the ΔHf⦵ of MgCl2 is -642Kjmol-1 , calculate the ΔH⦵lat (note: The construction of a complete Born–Haber cycle will not be assessed).
What are the factors that influence the strength of lattice enthalpy in an ionic compound?
A Born–Haber cycle is an application of Hess’s law, used to show energy changes in the formation of an ionic compound.
The cycle includes: ionization energies (table 8), electron affinities (8), lattice enthalpy (18), enthalpy of formation (12), and enthalpy of atomization (using sublimation and/or bond enthalpies: ΔH⦵AT typically given, but also use bond enthalpies on table 11).
Ex1 Answer: _2325 KJmol-1 (Check MSJ Chem Born-Haber cycles for explanation).
The lattice strength depends on the forces of attraction between the positive and negative ions. This electrostatic force depends on two factors: The magnitude of the charge on the ions. The distance between the ions (the sum of the ionic radii)