IB Chem Mini Exam Unit 13 - Descriptive Material
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
21 Terms
Distinguish between an Average rate and an Instantaneous Rate.
The average rate is the rate of the overall concentration of the reactant or the product over an average period of time. The instantaneous rate is the rate of the reactant or product at a specific time.
Name two reactions that are very fast and two that are very slow.
Two very fast reactions would be combustion in an engine and a nuclear chain reaction. Two reactions that would be very slow would be the rusting of iron and turning graphite into diamond.
For the reaction 24 + B + 2C → D + 2E, the rate law is: rate -k[A]^2[B]^1[C]^1
Which of the following statements is false:
a. the reaction is second order in [A]
b. the reaction is first order in [B]
c. the reaction is second order in [C]
d. the reaction is 4th order overall
c. the reaction is second order in [C]
For the reaction, 1A + 2B + 1C → 2D + 1E, the rate law is: rate =k[B]^2[C]^1
Which of the following statements is false:
a. the reaction is first order in [A]
b. the reaction is second order in [B]
c. the reaction is first order in [C]
d. the reaction is third order overall
a. the reaction is first order in [A]

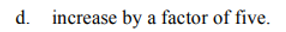

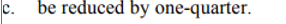

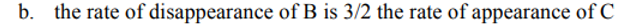
How are rate laws determined?
Rate Laws are determined experimentally
Do we use stoichiometric coefficients when determining rate laws?
No, because rate laws are determined experimentally and depend on the reaction mechanism rather than the stoichiometric coefficients in the balanced equation.
Why is it important to study kinetics?
Studying Kinetics is important becuase it allows us to understand the speed of which reactions takr place and aid us in drug design, food processing, and pollution control. Chemists or Chemical Enginneers might want to be able to figure out how to speed reactions up or find a new using kinetics.
What do the brackets mean in rate laws and rate expressions?
The brackets represent the molar concentration of reactant or product.
What are ways to experimentally determine reaction rates?
Reaction rates can be determined through spectrscopic means for solution, electrical conductance for ions, and pressure measurements for gases.
Can we determine rate laws without experimental data?
No, because rate laws must be determined experimentally since the reaction mechanism is not known from the balanced equation alone.
What do reaction rates depend on?
Temperature and the concentration of the reactants.
What is k? What does it depend on?
k is the rate constant and it only depends on temperature
Are rate laws written in terms of reactants or products?
Rate laws are written in terms of reactants
Is stoichiometry important in writing rate expressions?
Yes
If we plot concentration(molarity) vs time what is the curve?
An exponential curve
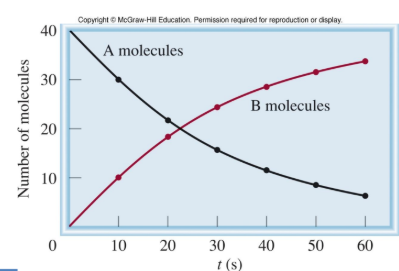
What must we plot to get a straight line?
To get a straight line on a graph you must plot the Rate (M/s) vs. the concentration of the reactant (M)
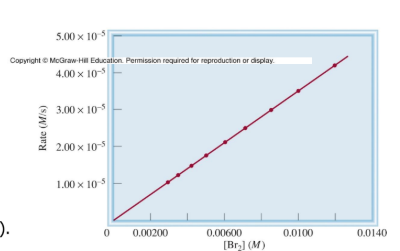
What happens to the rate if we double the concentration in this graph?
The Rate will double as the Rate is dependent on the concentration of the reactant
What happens to k if we double the concentration.
Nothing wil happen to k, as k is only dependent on Temperature.