2.4.1 - VSEPR Theory
0.0(0)
Card Sorting
1/6
Earn XP
Description and Tags
Last updated 12:06 PM on 11/3/22
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
7 Terms
1
New cards
Lone pairs
Valence electron pairs that are not bonded to any other atom.
2
New cards
VSEPR
Valence Shell Electron Pair Repulsion
3
New cards
Linear shape
One or two bonding regions; no lone pairs
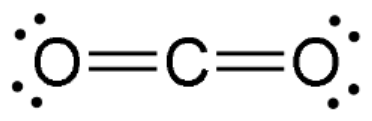
4
New cards
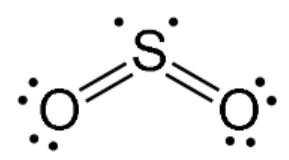
V-shaped
Two bonding regions; one or two lone pairs; always polar
5
New cards
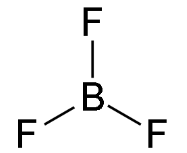
Trigonal planar-shaped
Three bonding regions; no lone pairs
6
New cards
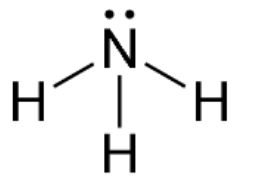
Trigonal pyramidal-shaped
Three bonding regions; one lone pair; always polar
7
New cards
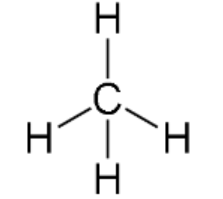
Tetrahedral-shaped
Four bonding regions; no lone pair