Chapter 10-17.2 for Midterm
1/286
Earn XP
Description and Tags
just merged all of my AP Chemistry notes from past tests and quizzes. can find specific chapters in my AP Chemistry folder
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
287 Terms
Boyle’s Law
the volume of a fixed quantity of gas is inversely proportional to its pressure; V = constant x 1/P or PV = constant
Charles Law
the volume of a fixed quantity of gas at constant pressure increases as the temperature increases; V = constant x T or V/T = constant
Gay-Lussac’s Law of combining volumes
at a given temperature and pressure, the volumes of gases which react are ratios of small whole numbers; the pressure and Kelvin temperature of a gas are directly proportional, provided the volume remains constant; P1/T1 = P2/T2
Combined Gas Law
relationship between pressure, volume, and temperature of a fixed amount of gas; combination of Boyle’s, Charles's, and Gay-Lussac’s laws; (P1V1)/T1 = (P2V2)/T2
Avogadro’s Hypothesis
equal volumes of gas at the same temperature and pressure will contain the same number of molecules
Avogadro’s Law
the volume of gas at a given temperature and pressure is directly proportional to the number of moles of gas; 22.4 L of any gas at 0 degrees C contain 6.02 × 1023 gas molecules; V = constant x n
Gases
composed of nonmetallic elements, simple moleculars formulas, and low molar masses; made up of molecules or atoms that are arranged without structure; no fixed shape or volume
Vapor
substances that are liquids of solids under ordinary conditions that also exist in the gaseous state; H2O exists as liquid water, solid ice, or water vapor
Iiquid
has a definite volume but no definite shape; made up of atoms or molecules that are connected by bonds and their particles can flow freely; less rigid than solids but more rigid than gases
Solid
a substance that has definite shape and volume; particles are arranged in a specific arrangement; firm or hard
Properties of Gas
expand spontaneously (to fill a container); highly compressible; form homogeneous mixtures; nonmetallic elements; different chemical properties
Absolute Zero
0 K or -273.15 degrees C; William Thomson proposed an absolute-temperature scale known as Kelvin scale
Density as it relates to gases
d = (nM)/V = (PM)/(RT); depends on its pressure, molar mass, and temperature; the higher the molar mass and pressure, denser the gas; higher temperature, less dense the gas; less dense gas will lie above a denser gas without mixing (hotter gas is less dense so it rises); molar mass of a gas = M = (dRT)/P
Kinetic Molecular Theory
the theory of moving molecules; pressure of a gas caused by collisions with container but its magnitude is determined by how often and how forcefully the collisions happend; proportional to temperature so at any temperature, same average kinetic energy; if absolute temperature is doubled, average kinetic energy of its molecules doubles; KE = ½ mu²
5 Parts of Kinetic Molecular Theory
random motion (gases consists of molecules in continuous random motion); negligible molecular volume (combined volume of all of the gas’ molecules is relative to its total volume); negligible forces (attractive and repulsive forces between gas molecules are insignificant); constant average kinetic energy(as temperature stays constant, the average kinetic energy of molecules does not change; energy can be transfered during collisions though); average kinetic energy proportional to temperature (proportional to absolute temperature; at any temperature, all gas molecules have same average kinetic energy)
Diffusion
spread of one substance throughout a space for a second substance; faster for light gas molecules; significantly slower than RMS speed; slowed by gas molecules colliding with each other
Effusion
escape of gas molecules through a tiny hole; Graham’s Law
Graham’s Law of Effusion
r1/r2 = sqrt(M2/M1); r1 and r2 - effusion of two gases; M1 and M2 - molar masses; a lighter gas has the higher effusion rate; rate of effusion is proportional to the rms speed (root mean square speed- speed of molecule having the same kinetic energy and average kinetic energy)
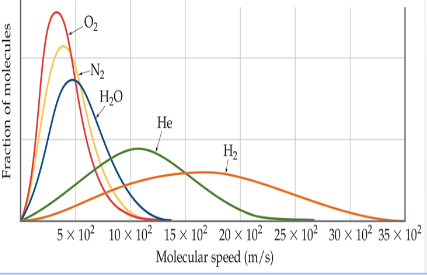
Maxwell Boltzmann
the distribution of speeds for a gas at a certain temperature; higher temperatures increase average particle speed; high molar mass, average particle speed decreases; average kinetic energy increases as temperature increases because temperature increase increases particle speed that is directly proportional to kinetic energy; molar mass does not affect average kinetic energy because increasing molar mass decreases particle speed which cancel out so kinetic energy stays the same
What causes deviations from the ideal gas law?
higher pressure = greater deviation → gas molecules get closer, intermolecular distance decreases so attractive forces take over; temperature increase = more ideal gas → because gas molecules move faster and apart so more energy is available to break intermolecular forces; increase with increasing molecular complexity (volume + attractive forces) and increasing mass (volume); volumes of real gases are larger and have smaller pressures of ideal gases
Ideal Gas
V = (nRT)/P; molecules do not interact with each other; molecules’ combined volume is much smaller than the volume the gas occupies
Standard Temperature and Pressure
0 C and 1 atm
Pressure (P)
force per unit; SI is pascals (Pa); bars (bar = 105 Pa); atmosphere (atm) and torr (torr or mmHg); barometer measures atmospheric pressure; manometer measures pressure of enclosed gases
Volume (V)
liters
Temperature
kelvins
Dalton’s Law of partial pressure
each gas exerts if present alone under same conditions; add all of the partial pressures up to make total pressure; partial pressure = mole fractions times total pressure
Mole Fraction
ratio of moles of one component of a mixture to the total moles of all components
root-mean-square (RMS) speed, urms
varies in proportion to the square root for the absolute temperature and inversely with the square root of the molar mass; = sqrt((3RT)/M) but most probably speed of a gas molecules is ump = sqrt((2RT)/M)
Mean free path
mean distance traveled between collisions; moving molecules has short path; collisions between molecules limit diffusion rate
as pressure increases, volume
decreases
as volume increase, pressure
decreases
as temperature increases, volume
increases
as pressure increases, n
increases
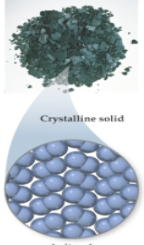
Particle arrangement in solids
closely packed in an ordered array; positions are essentially fixed; energies of particle-particle attraction are greater than kinetic energies of particles
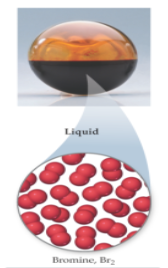
Particle arrangement in liquids
particles are closely packed but randomly orientated; retain freedom of motion; kinetic energies of particles similar to energies of particle-particle attraction
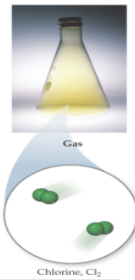
Particle arrangment in gas
particles are far apart; posses complete freedom of motion; kinetic energies of particles are greater than the energies of particle-particle attraction
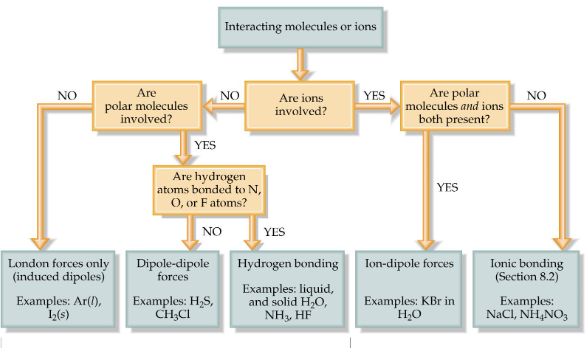
Ion-Dipole Forces
interaction between an ion and a dipole; a neutral, polar molecule (ex: water); strongest of all intermolecular forces (solutions ONLY)
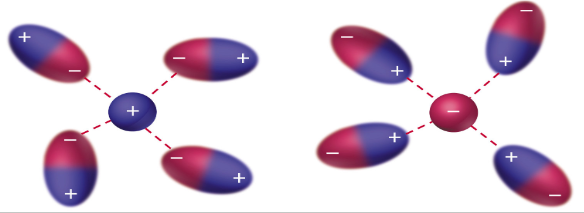
Dipole-Dipole Forces
between neutral polar molecules (oppositely charged ends of molecules attract); weaker than ion-dipole forces; increase with increasing polarity; strength of attractive forces is inversely related to molecular volume
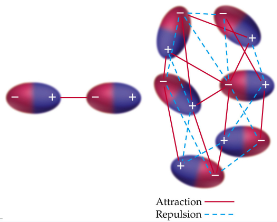
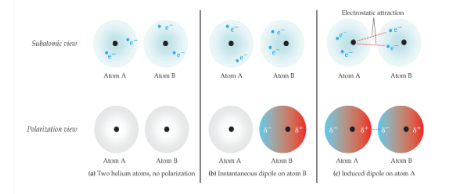
London Dispersion Forces
weakest of all intermolecular forces; two adjacent neutral, nonpolar molecules; the nucleus of one attracts the electrons of the other; electron clouds are distorted; instantaneous dipole; strength of forces is directly related to molecular weight; exist between all molecules; depend on the shape of the molecules; the greater the surface area available for contact, the greater the force is
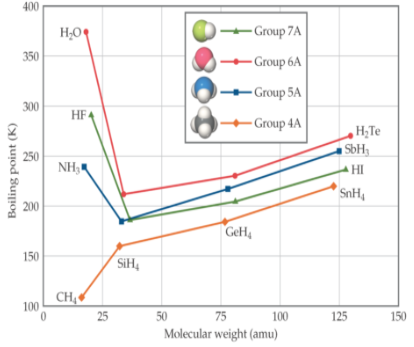
Hydrogen Bonding
special case of dipole-dipole forces; H-bonding requires H bonded to an electronegative element (F, O, N); boiling increases with increasing molecular weight (exception water)
Intermolecular Forces
London dispersion forces, dipole-dipole forces, hydrogen bonding, ion-dipole forces, ionic bonding
What is the effect of molar mass on IMFs?
increasing molar mass has stronger IMFs
What is the effect of structure on IMFs?
longer molecules have a greater surface area result in stronger IMFS
The stronger the attractive forces, the (boiling point and melting point)
the higher the boiling point of the liquid and the melting point of a solid
Temperature of boiling point increases as pressure
decreases
high altitude: low pressure so water boils at
lower temperature
liquids boil when the external pressure equals
the vapor pressure
Normal boiling point
Boiling point of a liquid at 1 atm
Vapor Pressure
pressure exerted when the liquid and vapor are in dynamic equilibrium; some molecules on the surface of a liquid have enough energy to escape to the gas phase, after some time the pressure of the gas will be constant at the vapor pressure (equilibrium)
Dynamic Equilibrium
the point when as many molecules escape the surface as strike the surface
Vapor Pressure increases nonlinearly with increasing
temperature (Clausius-Clapeyron Equation)
If equilibrium is never established then the liquid
evaporates; volatile substances (high VP) evaporate rapidly
the higher the temperature, the higher the average KE, the _____ the liquid evaporates
faster; (hot water is even faster than cold water)
Volatility
liquids that evaporate readily
What is the effect of IMFs on vapor pressure?
stronger the forces, the lower the vapor pressure; inverse relationship; fewer molecules will have enough KE to escape and substances with high vapor pressures are volatile, easily evaporate
What is the effect of surface area on vapor pressure?
no effect
Viscosity
resistance of a liquid to flow; molecules slide over each other
What is the effect of temperature on viscosity of a substances?
inverse relationship; viscosity decreases with increased temperature; increasing temperature increases energy and the velocity, so they interact for shorter time reducing internal friction and decreasing viscosity
What is the effect of molecular weight on viscosity of a substances?
viscosity increases with an increase in molecular weight; proportional
What is the effect of IMFs on viscosity of a substances?
the stronger the intermolecular forces, the higher the viscosity; proportional
Meniscus formation and characteristics
when adhesive forces are greater than cohesive forces, the water binds to the graduated cylinder creating a U-shaped meniscus; if the cohesive forces are greater, then the water binds to itself creating a curved downwards meniscus
Adhesive forces
bind molecules to a surface
Cohesive forces
bind molecules to each other
Surface Tension
amount of energy required to increase the surface area of a liquid
Phase Changes
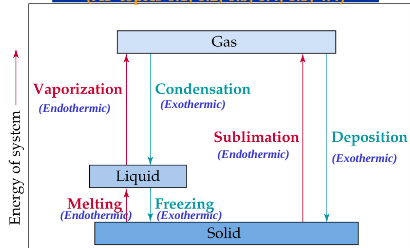
Generally the heat of fusion is less than
the heat of vaporization; takes more energy to completely separate molecules, than to partially separate them
What is the term for melting?
heat of fusion
What is the term for evaporation?
the heat of vaporization
Vaporization
endothermic; liquid → gas
Melting
endothermic; solid→liquid
sublimation
endothermic; solid→gas
condensation
exothermic; gas→liquid
freezing
exothermic; liquid→solid
deposition
exothermic; gas→solid
Heating curve
plot of temperature change vs. heat added; during a phase change, adding heat causes no temperature change (equilibrium); points calculate change in Hfus and change in Hvap
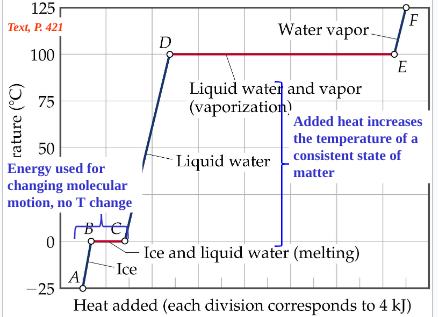
Gases are liquefied by increasing __________ at some temperature
pressure
critical temperature
the minimum temperature for liquefaction of a gas using pressure; high CT means strong intermolecular forces
Critical pressure
pressure required for liquefaction
Exothermic
transfers heat to the surroundings; feels hot
Endothermic
absorbs heat from surroundings; feels cold
Coulomb’s Law
the strength of the electrostatic force (attraction/repulsion) between two charged objects; higher charges, higher electrostatic forces. longer distances, lower electrostatic forces
Metallic solids
held together by a “sea” of collectively shared electrons
Ionic soldis
sets of cations and anions mutually attracted to each other (Coulomb’s Law)
Covalent-network soldis
joined by an extensive network of covalent bonds
molecular solids
discrete molecules held together by weak forces
Metal
group of cations suspended in a sea of electrons (electron-sea model)
Alloys
materials that contain more than one element and have characteristic properties of metals; employed to change the properties of certain metals
Substitutional alloys
second element takes the place for a metal atom; homogeneus mixture; components dispersed randomly and uniformly; atoms of solid occupy positions occupied normally by a solvent atom; 2 metallic components with similar atomic radii and chemical-bonding characteristics
Interstitial alloys
second element fills a space in the lattice of metal atoms; homogeneous mixtures; components dispersed randomly and uniformly; atoms of the solute occupy positions in the “holes” between solvent atoms; solute atoms need to have smaller bonding atomic radius than solvent atoms; its element is a nonmetal that makes covalent bonds with the metal atoms; harder, stronger, less ductile
Heterogeneous alloys
components not dispersed uniformly; components are not dispersed uniformly; properties depend on the composition and manner when a solid is formed from molten mixture; formed by rapid cooling are different from slow cooling of same mixture
Properties of Ionic Solids
very high melting and boiling points; quintessential crystals; charge is centered on the anions, electronic insulators; favorable Structures with hcation-anion distances as close as possible; CsCl structure, NaCl structure and Zince blende (ZnS) structure
cesium chloride (CsCl) structure; primitive cubic lattice
two atom-basis; center atom; no lattice point inside primitive unit cell; anions sit on the lattice points at the corners and cations sit in the center of the cell; surrounded by 8 atoms;
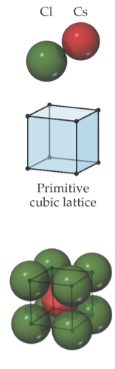
Sodium Chloride (NaCl); rock salt structure
face-centered cubic lattice; anions sit on lattice points that lie on the corners and faces of unit cell; cations are displaced from anions along the edge of the unit cell; each cation and anion are surrounded by six ions of the opposite type; octahedral coordination environment
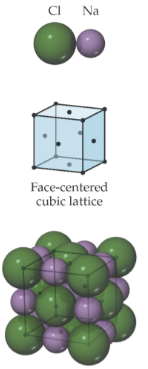
Zinc blende (ZnS)
face-centered cubic lattice; anions sit on the lattice points that lie on the corners and faces of the cell; cations are displaced from anions along the body diagonal of the cell; each cation and anion are surrounded by 4 of the opposite type; tetrahedral coordination geometry
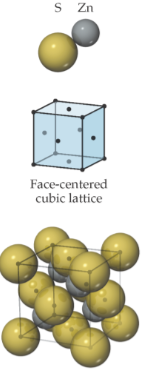
When to use the ionic structure based on ion size?
cation and anion are similar in size, large coordination # is favored so CsCl structure; relative size of cation gets smaller, coordination # drops from 8 to 6, sodium chloride structure; cation size decreases a lot, coordination # reduces from 6 to 4, zinc blende structure
Weak IMFs, solubility is
low (less polarity)
Large dipole movement, solubility is
increased (greater molecular polarity)
More hydrogen bonding, solubility is
increased (greater polarity)