orgo test 3 vocab
1/18
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
19 Terms
aprotic
cant hydrogen bond with itself/doesn't have any donor hydrogens (hydrogens attached to a N, O, or F atom)
protic
can hydrogen bond with itself/has donor hydrogens (hydrogens attached to a N, O, or F atom)
a ___ base =a better leaving group
weaker base
hybridization
Combining atomic orbitals (ex:sp3)
inductive effect
A change in electron density of an atom bc of the atoms around it in a molecule
polarizability
How easily a molec’s electron cloud can be distorted
solvation
Solvent molecules interact/ form bonds with ions in the solvent, stabilizing them
A ___ base = a better nucleophile
stronger base
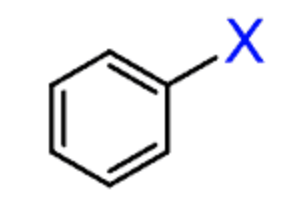
aryl halide
an alkyl halide with the halogen directly bonded to a ring
Vinyl halide
An alkyl halide with the halogen bonded to an atom with a double bond
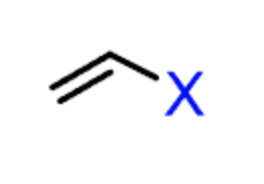
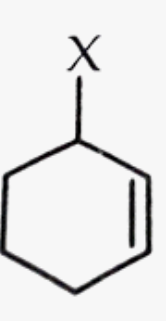
Allyl halide
alkyl halide where the halogen is 1 carbon away from a double bond
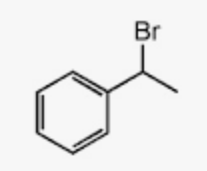
benzyl halide
alkyl halide where the halogen is 1 carbon away from a benzene ring
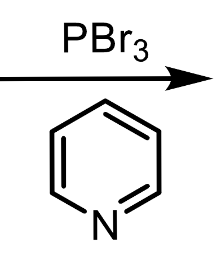
the only reagents you should use for secondary alcohols
phosphorus tribromide and pyridine
Williamson ether synthesis
Sn2 reaction that turns an alcohol and a primary alkyl halide onto an ether
what solvent you need to use in Williamson ether synthesis
NaH
What reactions is NaOCH3 used in?
acid/base reactions, SN2, E2
What reactions is KOtBu used in?
E2 reactions, SN2 only with BrCH3
What reactions is pyridine used in?
Williamsons ether synthesis with PBr3 or PCl3, acts as base in primary and secondary alcohol reactions, solvent
What reactions is NaH used in?
Williamsons ether synthesis, deprotonation