Physics 2 Midterm 1: iClicker
0.0(0)
0.0(0)
Card Sorting
1/23
Earn XP
Last updated 10:28 PM on 2/20/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
24 Terms
1
New cards
A sample of a low-density gas is initially at room temperature and has pressure p0. The gas is warmed at constant volume until the pressure is 2p0. Compared to the initial Celsius temperature of the gas, the final Celsius temperature is
greater by a factor of more than 2
2
New cards
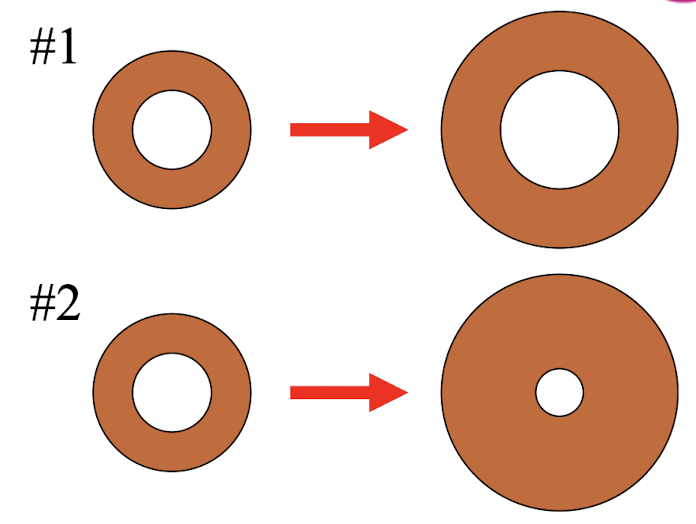
A solid object has a hole in it.
Which of these illustrations
more correctly shows how the
size of the object and the hole
change as the temperature
increases?
Which of these illustrations
more correctly shows how the
size of the object and the hole
change as the temperature
increases?
illustration #1
3
New cards
A pitcher contains 0.50 kg of liquid water and 0.50 kg of ice at 0°C. You let heat flow into the pitcher until there is 0.75 kg of liquid water and 0.25 kg of ice. During this process,
the temperature of the ice-water mixture remains the same.
4
New cards
A chair has a wooden seat but metal legs. The chair legs feel colder to the touch than does the seat. Why is this?
The metal has a higher thermal conductivity than the wood.
5
New cards
A quantity of an ideal gas is contained in a balloon. Initially the gas temperature is 27°C. You double the pressure on the balloon and change the temperature so that the balloon shrinks to one-quarter of its original volume. What is the new temperature of the gas?
–123°C
6
New cards
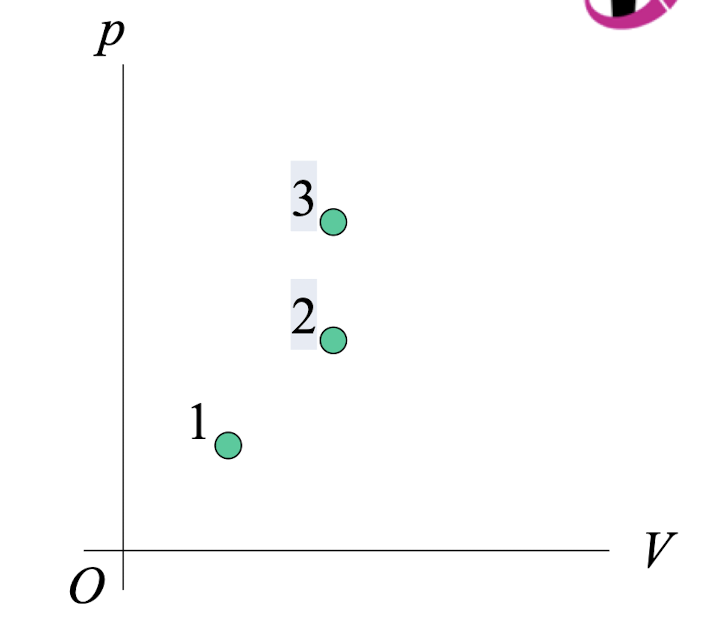
This p-V diagram shows three possible states of a certain amount of an ideal gas. 3
Which state is at the highest 2
temperature?
Which state is at the highest 2
temperature?
state #3
7
New cards
Consider two specimens of ideal gas at the same temperature. The molecules in specimen #1 have greater molar mass than the molecules in specimen #2. How do the rms speed of molecules (v rms) and the average translational kinetic energy per molecule (KE) compare in the two specimens?
V rms is greater in specimen #2; KE is the same in both specimens.
8
New cards
Consider two specimens of ideal gas at the same temperature. Specimen #1 has the same total mass as specimen #2, but the molecules in specimen #1 have greater molar mass than the molecules in specimen #2. In which specimen is the total translational kinetic energy of the entire gas greater?
specimen #2
9
New cards
You have a quantity of ideal gas in a cylinder with rigid walls that prevent the gas from expanding or contracting. If you double the rms speed of molecules in the gas, the gas pressure
increases by a factor of 4.
10
New cards
You have 1.00 mol of an ideal monatomic gas and 1.00 mol
of an ideal diatomic gas whose molecules can rotate. Initially
both gases are at room temperature. If the same amount of
heat flows into each gas, which gas will undergo the greatest
increase in temperature?
of an ideal diatomic gas whose molecules can rotate. Initially
both gases are at room temperature. If the same amount of
heat flows into each gas, which gas will undergo the greatest
increase in temperature?
the monatomic gas
11
New cards
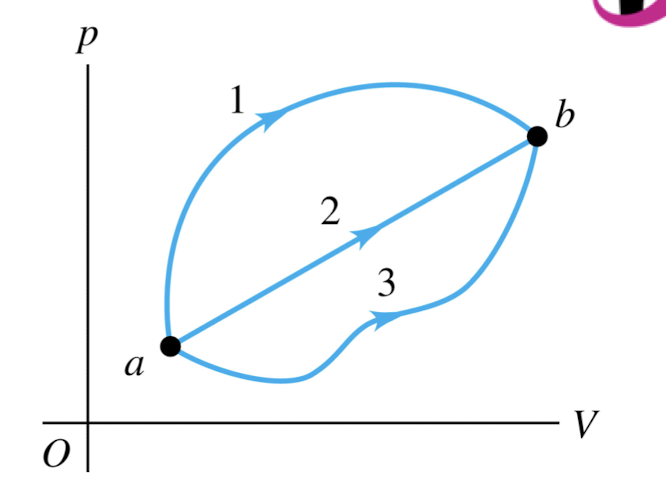
A system can be taken from state a to state b along any of the three paths shown in the p-V diagram. If state b has greater internal energy than state a, along which path is the absolute value |Q| of the heat transfer the greatest?
path 1
12
New cards
In an isothermal expansion of an ideal gas, the amount of heat that flows into the gas
equals the amount of work done by the gas.
13
New cards
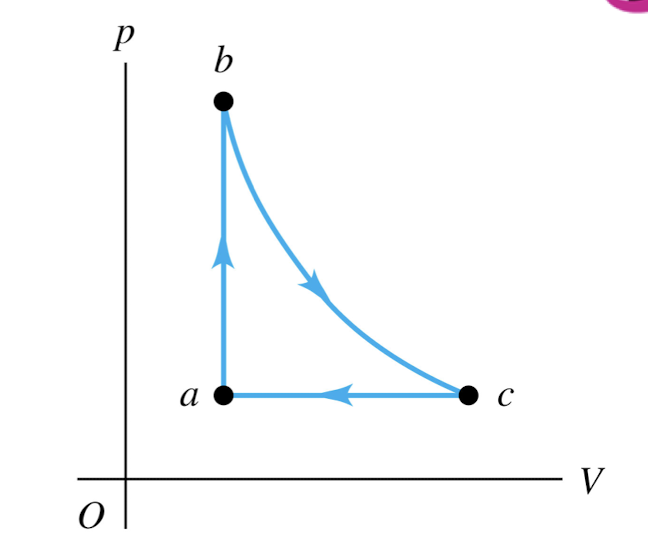
An ideal gas is taken around the
cycle shown in this p-V diagram,
from a to b to c and back to a.
Process b → c is isothermal.
For this complete cycle,
cycle shown in this p-V diagram,
from a to b to c and back to a.
Process b → c is isothermal.
For this complete cycle,
Q > 0, W > 0, and ∆U = 0
14
New cards
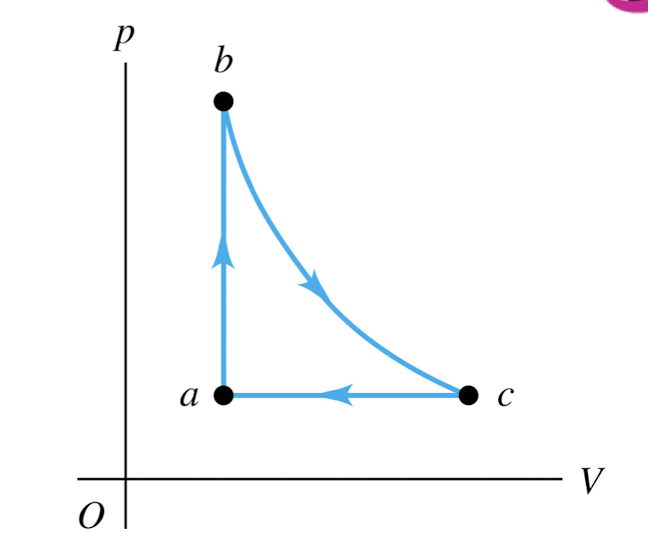
An ideal gas is taken around the cycle shown in this p-V diagram, from a to b to c and back to a. Process b → c is isothermal. For process a → b,
Q > 0 and ∆U > 0
15
New cards
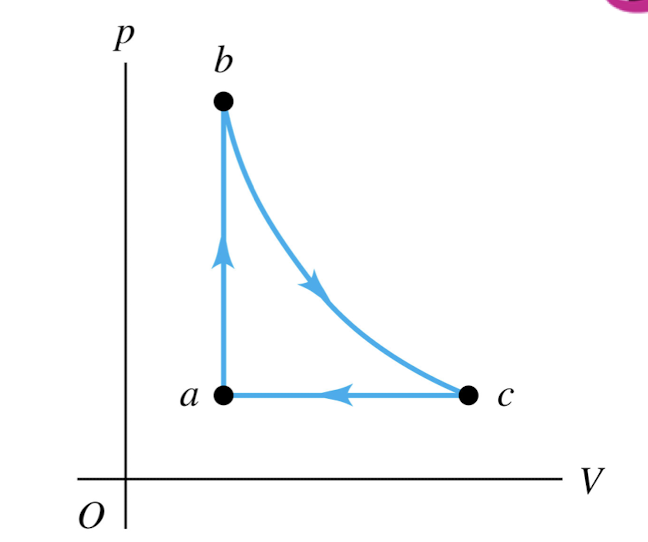
An ideal gas is taken around the cycle shown in this p-V diagram, from a to b to c and back to a. Process b → c is isothermal. For process b → c,
Q > 0 and ∆U = 0
16
New cards
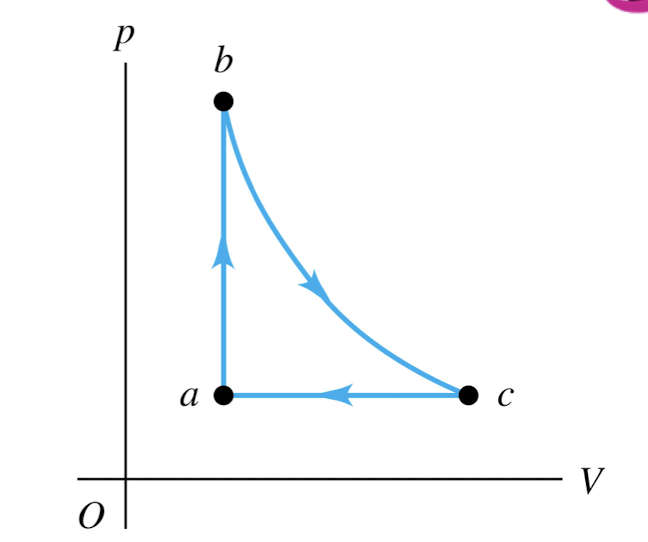
An ideal gas is taken around the cycle shown in this p-V diagram, from a to b to c and back to a. Process b → c is isothermal. For process c → a,
Q < 0 and ∆U < 0
17
New cards
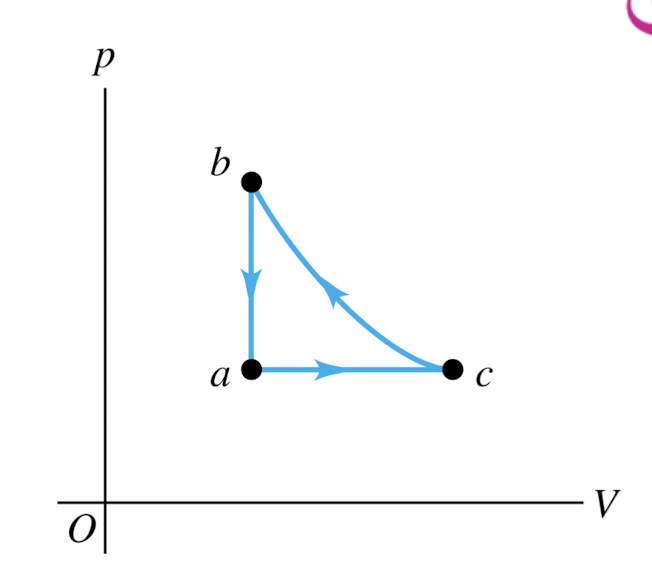
An ideal gas is taken around the cycle shown in this p-V diagram, from a to c to b and back to a.
Process c → b is adiabatic.
For process c → b,
Process c → b is adiabatic.
For process c → b,
Q = 0, W < 0, and ∆U > 0
18
New cards
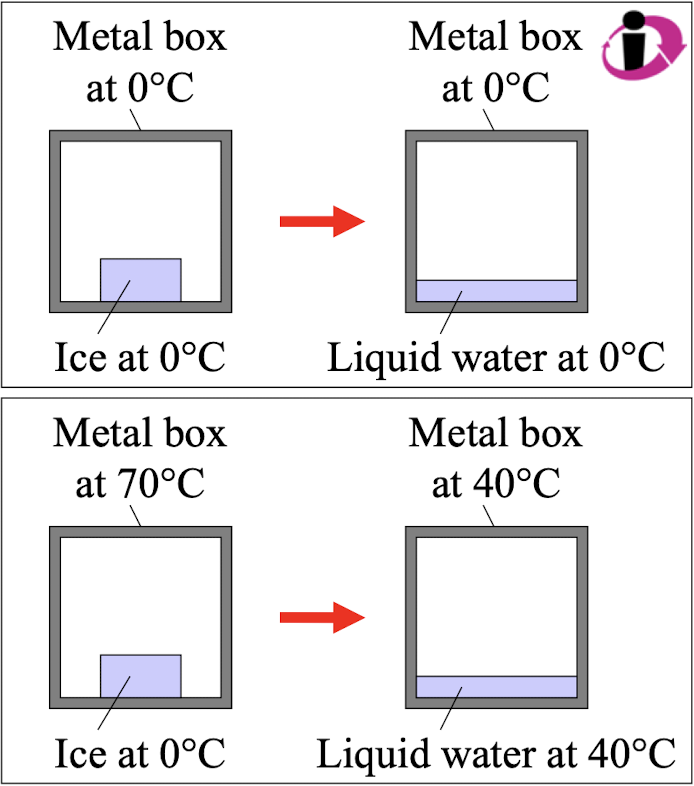
Which statement about these two thermodynamic processes is correct?
The upper one is reversible and the lower one is irreversible
19
New cards
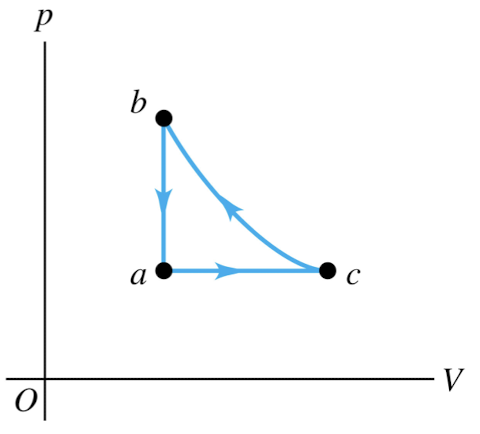
An ideal gas is taken around the
cycle shown in this p-V diagram,
from a to c to b and back to a.
Process c → b is adiabatic.
Which of the processes in this
cycle could be reversible?
cycle shown in this p-V diagram,
from a to c to b and back to a.
Process c → b is adiabatic.
Which of the processes in this
cycle could be reversible?
c → b
20
New cards
During one cycle, an automobile engine takes in 12,000 J of heat and discards 9000 J of heat. What is the efficiency of this engine?
25%
21
New cards
During one cycle, an automobile engine with an efficiency of 20% takes in 10,000 J of heat. How much work does the engine do per cycle?
2000 J
22
New cards
A copper pot at room temperature is filled with room- temperature water. Imagine a process whereby the water spontaneously freezes and the pot becomes hot. Why is such a process impossible?
It violates the second law of thermodynamics.
23
New cards
A Carnot engine takes heat in from a reservoir at 400 K and discards heat to a reservoir at 300 K. If the engine does 12,000 J of work per cycle, how much heat does it take in per cycle?
48,000 J
24
New cards
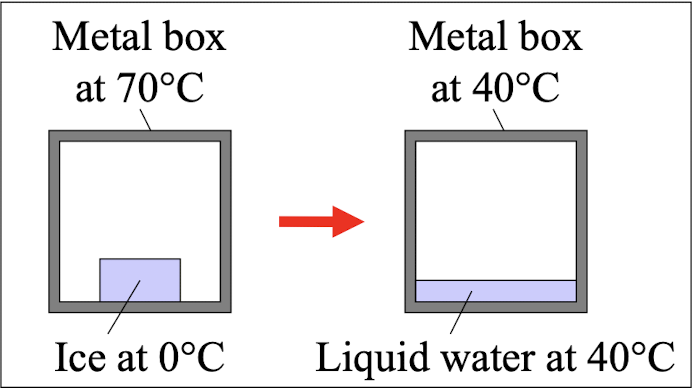
You put an ice cube at
0°C inside a large metal
box at 70°C. The ice
melts and the entropy of
the ice increases. Which
statement is correct?
0°C inside a large metal
box at 70°C. The ice
melts and the entropy of
the ice increases. Which
statement is correct?
Entropy of the metal box decreases; total entropy increases.