Functional Groups, Geometry, & Hybridization
1/60
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
61 Terms
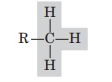
Methyl
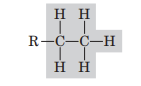
Ethyl
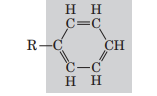
Phenyl
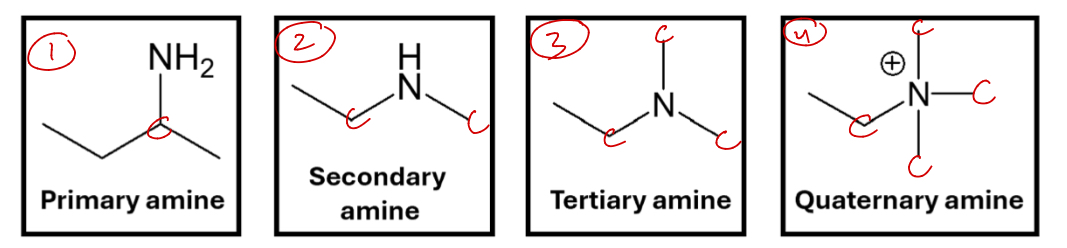
Amine (Amino)
Derived from ammonia NH3
primary, secondary, tertiary, quaternary
Amino = molecule that contains an amine group
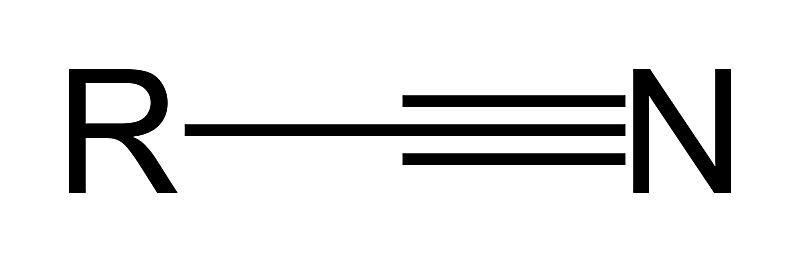
Nitrile (Cyano)
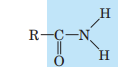
Amide (Amido)
Nitro compounds
Contains nitro NO2 group
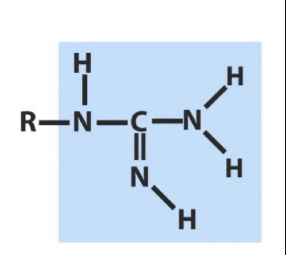
Guanidino
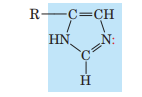
Imidazole
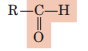
Aldehyde (Carbonyl)
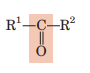
Ketone (Carbonyl)
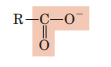
Carboxyl
Carboxylic acid = molecule that contains a carboxyl group and behaves as an acid (can donate H⁺)
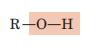
Hydroxyl (Alcohol)
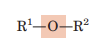
Ether
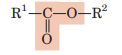
Ester
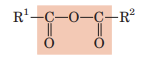
Anhydride
2 carboxylic acids
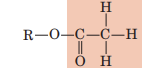
Acetyl
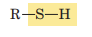
Sulfhydryl (Thiol)

Disulfide
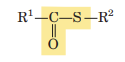
Thioester
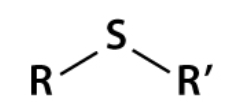
Thioether
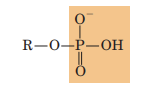
Phosphoryl
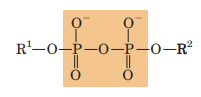
Phosphoanhydride
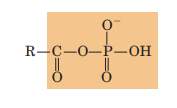
Mixed anhydride (acyl phosphate)
carboxylic acid and phosphoric acid
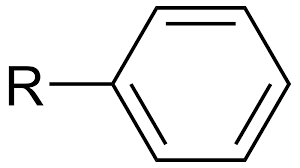
Aromatic (Arene)
6-carbon ring with 3 double bonds
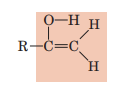
Enol
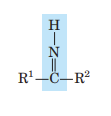
Imine
Alkane
hydrocarbon with C—C single bond
Alkene
hydrocarbon with C—C double bond
Alkyne
hydrocarbon with C—C triple bond
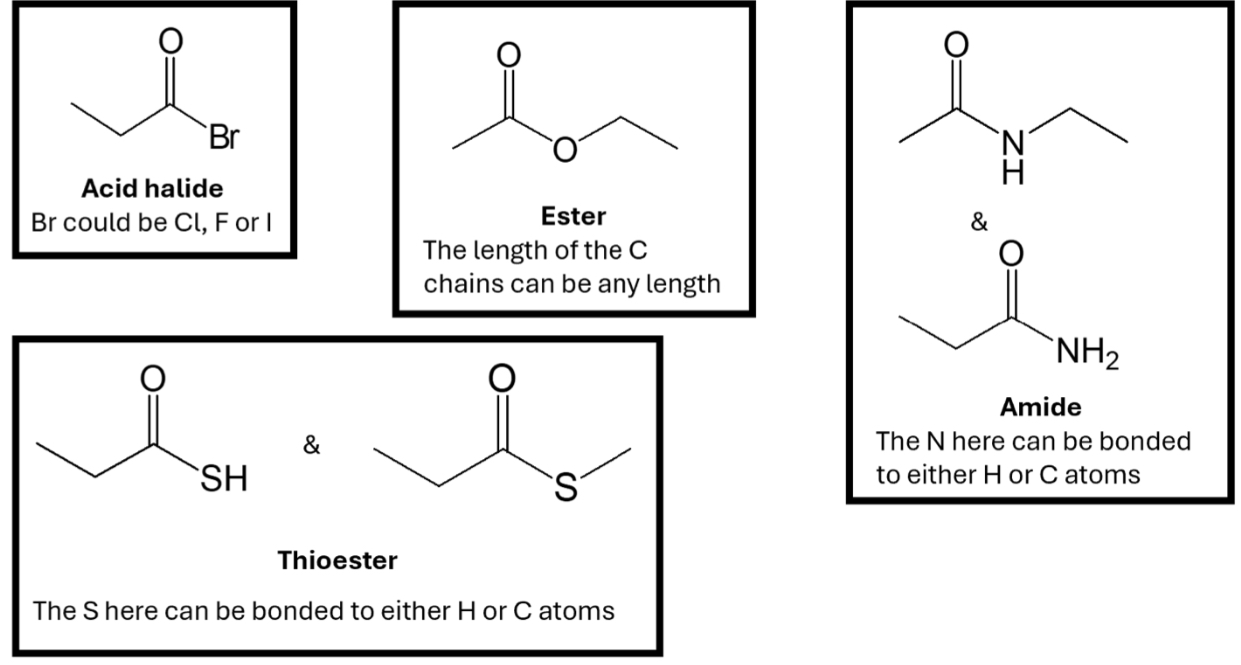
Carboxylic acid derivatives
Carbonyl bound to EN atom
Ex. Acid halides, Esters, Amide, Thioester
Compounds containing Sulfur
Thiols, thiothers, thioesters, sulfonic acids, sulfoxide, sulfone
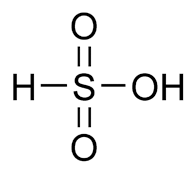
sulfonic acid
similar to carboxylic acids
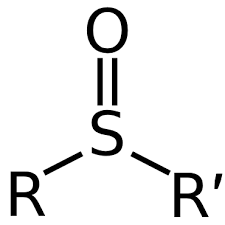
Sulfoxide
mildly oxidized (gained some oxygen)
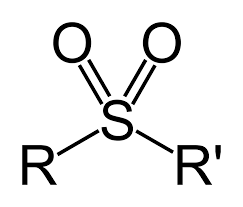
Sulfone
fully oxidized
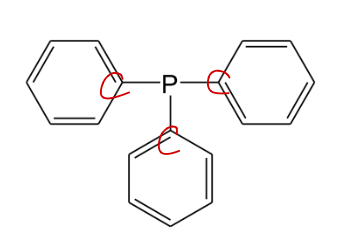
Phosphine
Phosphorus bonded to 3 different carbons
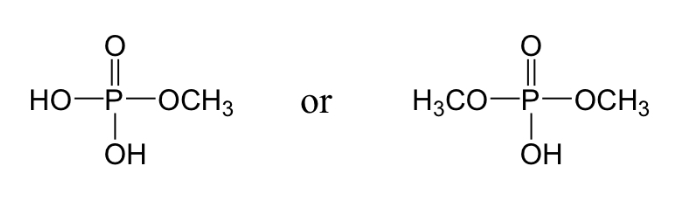
Phosphate ester
2 e groups & 0 lone pairs
Linear
180 degrees
sp
3 e groups, 0 lone pairs
Trigonal planar
120 degrees
sp2
3 e groups, 1 lone pair
electron geometry = Trigonal planar
molecular geometry = Bent
<120 degrees
sp2
4 e groups, 0 lone pairs
Tetrahedral
109.5 degrees
sp3
4 e groups, 1 lone pair
electron geometry = Tetrahedral
molecular geometry = Trigonal pyramidal
<109.5 degrees
sp3
4 e groups, 2 lone pairs
electron geometry = Tetrahedral
molecular geometry = Bent
<109.5 degrees
sp3
5 e groups, 0 lone pairs
Trigonal bipyramidal
120 and 90 degrees
sp3d
5 e groups, 1 lone pair
electron geometry = Trigonal bipyramidal
molecular geometry = seesaw
<120 and <90 degrees
sp3d
5 e groups, 2 lone pairs
electron geometry = Trigonal bipyramidal
molecular geometry = T-shape
<90 degrees
sp3d
5 e groups, 3 lone pairs
electron geometry = Trigonal bipyramidal
molecular geometry = Linear
180 degrees
sp3d
6 e groups, 0 lone pairs
Octahedral
<90 degrees
6 e groups, 1 lone pair
electron geometry = Octahedral
molecular geometry = square pyramidal
<90 degrees
6 e groups, 2 lone pairs
electron geometry = Octahedral
molecular geometry = Square planar
90 degrees
6 e groups, 3 lone pairs
electron geometry = Octahedral
molecular geometry = T-shape
<90 degrees
6 e groups, 4 lone pairs
electron geometry = Octahedral
molecular geometry = Linear
180 degrees