Topic 1A - Molecules of Life
1/72
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
73 Terms
Monomers
Biological compounds which exist singularly, they aren’t linked to any other molecule
Polymers
Large and complex molecules which are comprised of monomers linked together. Most carbs, proteins and lipids are polymers. They are more useful as they’re unique with many different properties, making diversity of life possible.
Example drawings of a monomer and polymer
Ethene and polyethene
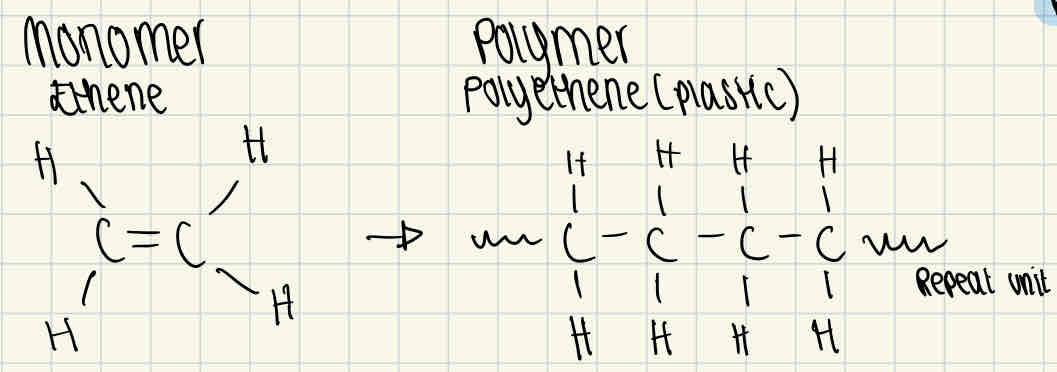
Making polymers
Formed through condensation, this is a dehydration reaction as water is lost. This chemically bonds the monomers with a glycosidic bond to create a polymer.
Breaking down polymers
Broken down through hydrolysis, this uses a water molecule to break the chemical bond which reverses the condensation reaction. This separates polymers back into monomers.
Carbohydrates
These are sugars and starches. They are the main source of energy in the body. There are 3 types of carbohydrates: monosaccharides, diasaccharides and polysaccharides.
Saccharide
This means sugar. The general formula is (CH₂)n where n = >3
Monosaccharides
The simplest sugars, they are building blocks for carbohydrates. All monomer carbs are made up of monosaccharides, some examples are glucose, fructose and galactose. These cannot be hydrolysed into smaller components.
What elements are carbs made of?
Carbon, hydrogen and oxygen.
Glucose
Glucose is a hexose sugar, which is a monosaccharide with six carbon atoms in each molecule.
Alpha glucose (a glucose) formula
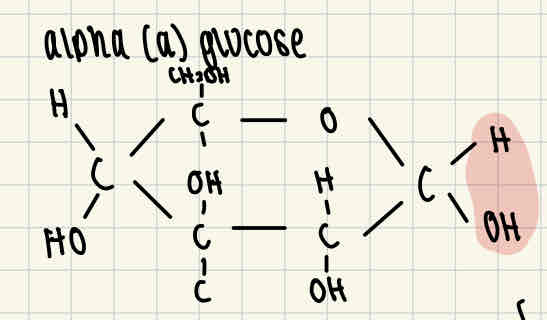
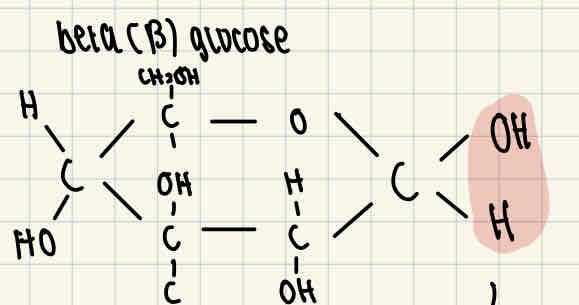
Beta glucose (ß glucose) formula
The difference between alpha and beta glucose is that the H and OH right end of the molecule are reversed.
Isomers
Molecules with the same formula which are connected in different ways, for example a and ß glucose.
Diasaccharides
Formed when two monomers join together, through a condensation reaction. A glycosidic bond is formed between the two monomers as water is released.
Examples of diasaccharides
Maltose, sucrose, lactose. For example, two a-glucose are joined together to form maltose.
Polysaccharides
Polymeric carbohydrate molecules composed of long. Hains of monosaccharide units which are bound by glycosidic bonds. General formula: Cₓ(H₂O)ᵧ where x = a large number between 200 and 2500.
Storage polysaccharides
Reservoirs of energy in the human body, they can be broken down and used (metabolised) when necessary. For example, starch.
Stuctural polysaccharides
Helps to upkeep the structure of a living organism. For example, cellulose forms important cells walls in plants which maintain their structure. Additionally, provides us with fibre to aid digestion.
How to test for sugars
Benedict’s test, sugars is a general term for monosaccharides and diasaccharides.
Reducing sugars procedure
Add blue Benedict’s reagent to a sample and bring this sample to a boil.
If the sample stays blue, then there are no reducing sugars present.
If the sample goes from green to yellow to orange and eventually has brick red precipitate = reducing sugar is present.
The higher the concentration of reducing sugars, the further the colour changes.
This can be measured using a colorimeter.
Non-reducing sugars procedure.
Some samples are negative for reducing sugar, but these may still contain non-reducing sugars.
To test this, you must break the sample down into monosaccharides by adding hydrochloric acid to a fresh sample and bringing it to a boil.
Then this is neutralised by adding sodium hydrocarbonate.
Then the Benedict’s test is carried out as normal, with brick red being the indicator for non-reducing sugars.
Polysaccharides
These are carbohydrates, made from large chains of monosaccharides (monomers) bonded together.
How are polysaccharides formed?
Polysaccharides are formed when more than 2 monosaccharides are joined through a condensation reaction. For example, a-glucose (a monosaccharide) is joined to 3 other a-glucose using glycosidic bonds to form amylose (a polysaccharide).
Breakdown of polysaccharides
Polysaccharides can be broken down to their consistent monosaccharides using hydrolysis, this is when H₂O is added. For example, amylose can be hydrolysed into a-glucose.
Functions of polysaccharides - Starch
Plants get energy from glucose and store extra glucose as starch, which is broken down to release glucose when necessary. Starch is insoluble and doesn’t affect water potential, this means that water can’t enter through osmosis to make it swell, making it good for storage.
Starch structure
Starch is a mix of two polysaccharides of a-glucose (amylose and amylopectin)
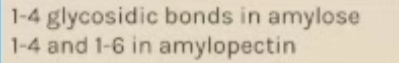
Starch - amylose
Amylose is a long and unbranched chain of a-glucose. The angles of the glycosidic bonds give it a coiled structure, making it compact which is ideal for storage.
Starch - amylopectin
Long, branched chain of a-glucose. The side branches allow enzymes that break it down to access the glycosidic bonds quickly, this means glucose can be released quickly.
Functions of polysaccharide - glycogen
Animal cells also get energy from glucose, however, excess glucose is instead stored as glycogen which is another polysaccharide of a-glucose.
Glycogen structure
The structure of glycogen is similar to amylopectin, but with far more side branches. This means that glucose can be released very quickly as the glycosidic bonds can be accessed and broken, while still remaining compact.
Functions of polysaccharides - Cellulose
Cellulose provides strong structural support for cells, for example it is used in plant cells to uphold the structure of a plant.
Cellulose structure
Cellulose is made up of long, unbranched chains of ß-glucose. When ß-glucose bonds, they create straight chains of cellulose. These chains are linked together by hydrogen bonds to form strong fibres called microfibrils.
Test for starch procedure
Iodine test
Add iodine solution (which has been dissolved in potassium iodide) to the sample
If starch is present, the sample changes from a browny-orange colour to a blue-black colour.
No colour change indicates there’s no starch present.
Lipids
Lipids are different to carbs/protein as they’re not polymers. Lipids are made from a variety of components but they all contain hydrocarbons (molecules made up of only hydrogen and carbon). The other components they’re made up is dependant on their function.
Triglycerides
This is a type of lipid which is made up of 1 molecule of glycerine and 3 fatty acids attached to it.
Fatty acids have a long tail, which is made up of hydrocarbons.
This tail is hydrophobic (repels water molecules), making lipids insoluble in water.
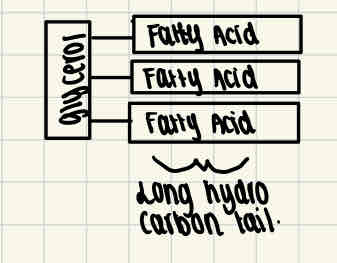
Formation of triglycerides
Triglycerides are formed through condensation reactions, an ester bond is formed between two molecules which releases water. This happens at least twice more to form a triglyceride.
Fatty acids structure
All fatty acids consist of the same basic structure, shown in the image. However, the structure of the hydrocarbon tail varies between saturated/unsaturated fatty acids.
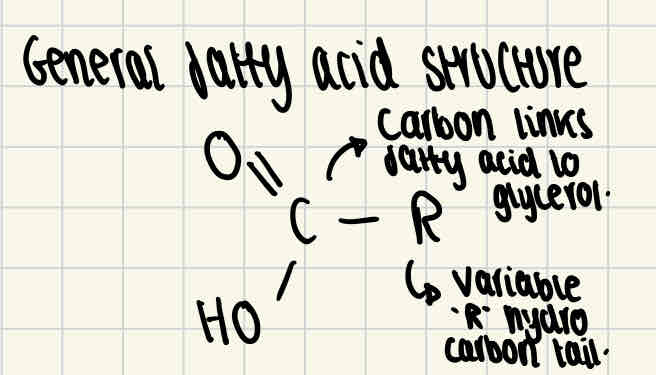
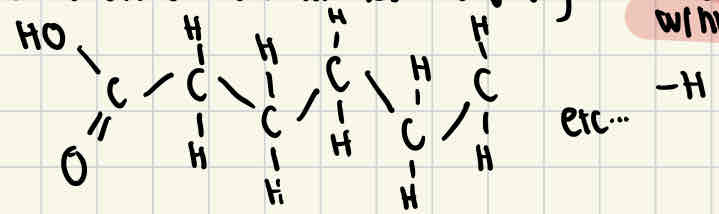
Saturated fatty acid structure
Saturated fatty acids don’t have any double bonds between carbon atoms, this means that the fatty acid is fully saturated with hydrogen
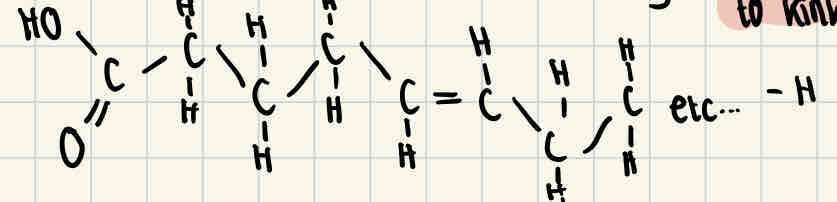
Unsaturated fatty acids
Unsaturated fatty acids do have double bonds between some of the carbon atoms, this causes the chain to kink and means that the fatty acid isn’t fully saturated with hydrogen.
Function of triglycerides
Triglycerides are used as energy storage molecules. Their structure is designed to carry out this function
The long hydrocarbon tail of fatty acids contains a lot of chemical energy, therefore a lot of energy is released when they’re broken down.
Triglycerides are insoluable in water, so they don’t affect the water potential of a cell. Therefore water can’t enter to make them swell, allowing them to be compact.
To repel water, the triglycerides bundle together, the hydrophobic tails face inward to shield themselves from water using their glycerol heads.
Phospholipids structure
This is another type of lipid which is found in the cell membrane. Structurally they are similar to triglycerides except one of the fatty acid molecules is replaced with a phosphate group. The phosphate group is hydrophilic (attracts water) while the fatty acids are hydrophobic.
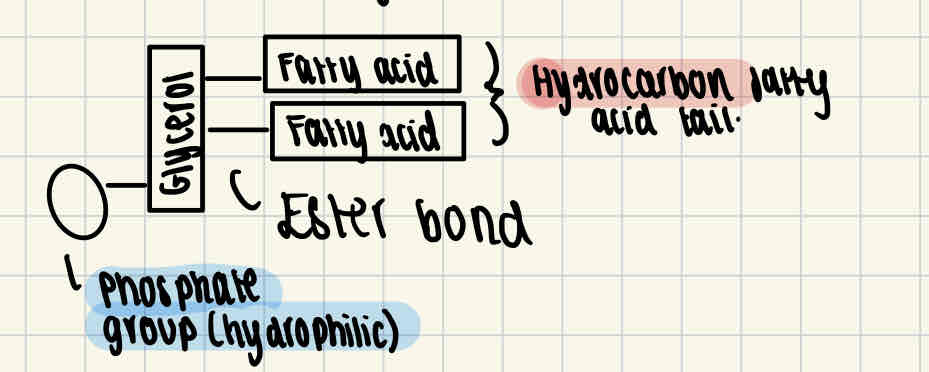
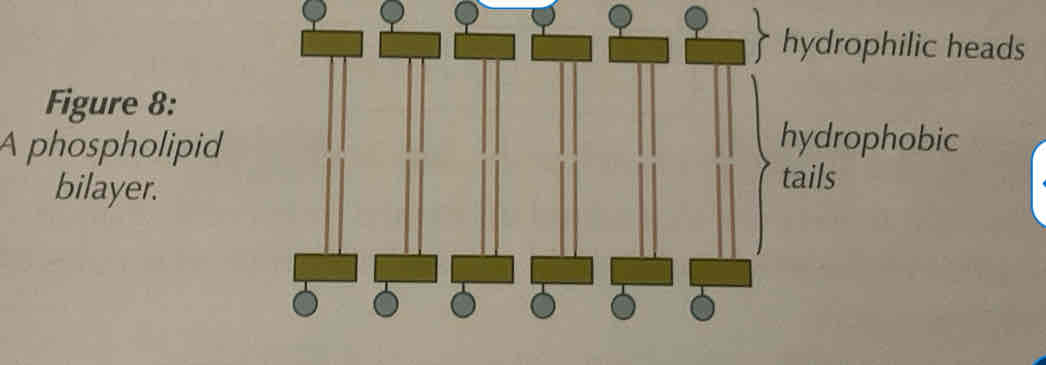
Function of phospholipids
Phospholipids act as the bilayer of cell membranes, which means the control what leaves and enters the cell.
Phospholipids will form a double layer with their heads facing outward to the water on each side (one side to the outside of the cell, one side to the inside)
The centre of the bilayer remains hydrophobic due to the fatty acids and glycerol, so the membrane acts as a barrier to these substances.
However, the head remains hydrophilic as it is the phosphate group.
Test for lipids
Emulsion test
Shake the test sample with ethanol for about 1 minute, then pour this solution into a test tube of water.
Lipids will show up as a milky emulsion, the more visible this is, the higher concentration of lipids in the sample.
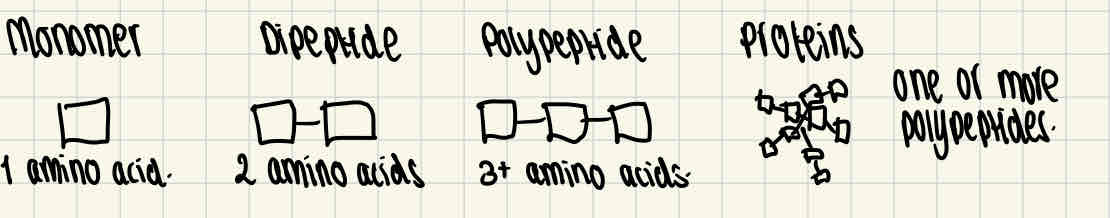
Proteins
Proteins are made up of the monomer, amino acids. They are made from one or more polypeptides of amino acids.
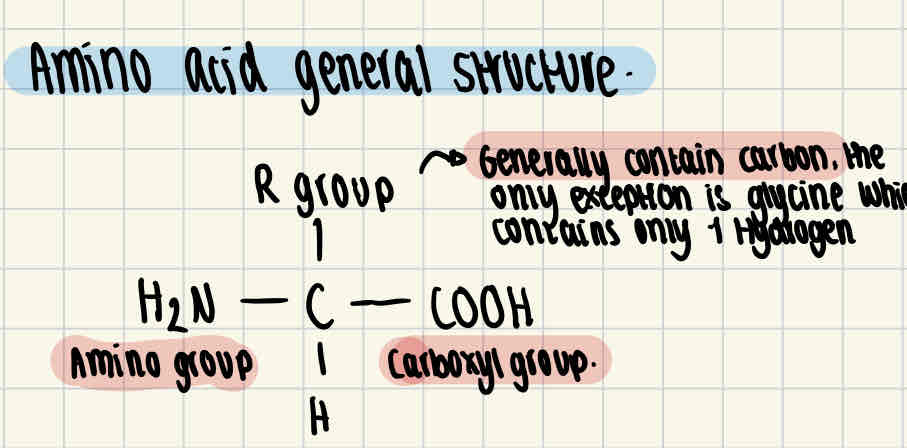
Amino acid general structure
Amino acids contain an amino group, a carboxyl group, an R group and a hydrogen atom.
Formation of di/polypeptides
Amino acids are linked by condensation reactions, which form dipeptides or polypeptides. During this reaction, a molecule of water is released and the bonds formed between the amino acids are called peptide bonds.
Break down of amino acids
Amino acids are broken down through hydrolysis, where a molecule of water is used to break the peptide bonds.
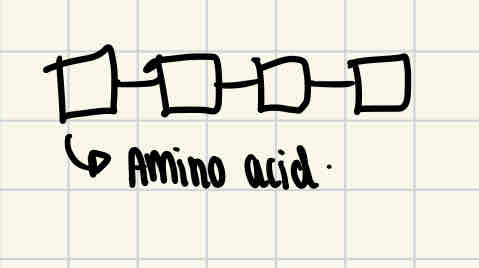
Protein structural levels - primary structure
This is the sequence of amino acids in a polypeptide chain
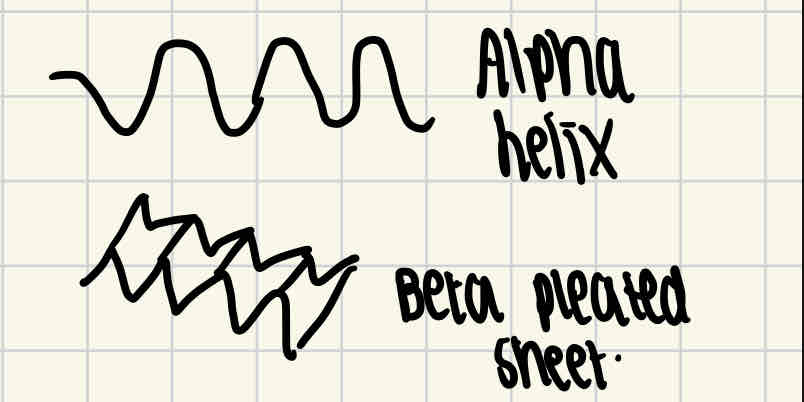
Protein structural levels - secondary structure
Hydrogen bonds are formed between the amino acid, which change the straight shape into a more kinked shape. This either causes it to coil into an alpha helix or a fold into a beta pleated sheet.
Protein structural levels - Tertiary structure
As more hydrogen and ionic bonds are formed between different parts of the polypeptide chain, this causes the coiled/folded chains to become further coiled.
Disulphide bridges are formed when two molecules of amino acid cysteine come close together, as the sulfur atom in one of the cysteine bonds to the sulfur atom in the other.
For proteins made up of a single polypeptide chain, the tertiary structure is their final 3D structure.
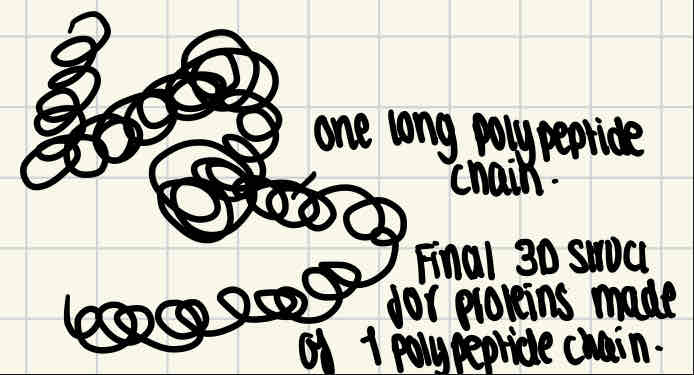
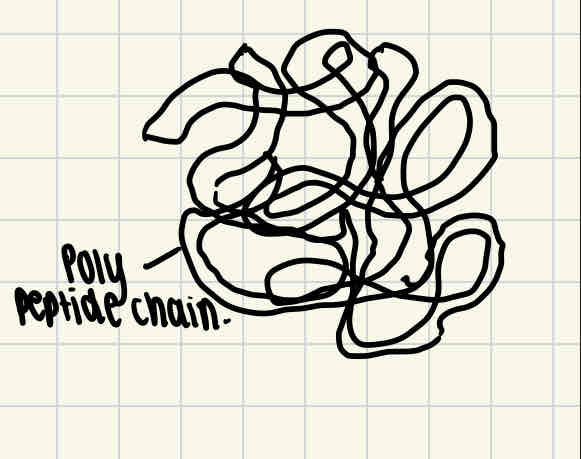
Protein structural levels - quaternary structure
Some proteins are made of several different polypeptide chains held together by bonds. The quanternary structure is the way these chains are assembled together to form a protein final 3D structure.
4 examples of proteins in life
A protein’s shape determines its function as they’re specialised to carry out specific jobs.
Enzymes, antibodies, transport proteins and structural proteins
Protein examples - enzymes
Enzymes are roughly spherical due to the tight folding of polypeptide chains. They’re soluble and have roles in digestion (e.g. lock and key shape), they also help to synthesise larger molecules.
Protein examples - antibodies
Antibodies are found in the blood and involved in the human body’s immune response. They’re made up of two light (short) polypeptide chains and two heavy (long) polypeptide chains.
Protein examples - transport proteins
Haemoglobin is an example of a transport protein, it has a compact shape and is a soluble protein which makes it great for transporting oxygen around the body.
Protein examples - structural proteins
Structural proteins consist of long polypeptide chains which lie parallel to each other, with cross links in between them, this makes them very physically strong. For example, collagen has 3 polypeptide chains which are tightly coiled together, this makes it a great supportive tissue in animals.
Test for protein
Biuret test for proteins
Test solution must be alkaline, so first a few drops of sodium hydroxide solution are added.
After this, copper Sulfate solution is added which has a blue colour.
If the solution turns purple, there is protein present and if it stays blue there is no protein present.
What are enzymes?
Enzymes are proteins which are biological catalysts, which mean they speed up metabolic reactions.
Both at a cellular level (respiration) and for the whole body (digestion.)
Enzymes can also affect structure, for example enzymes are involved in the production of collagen which is in connective tissues.
Enzymes action can be intracellular (in the cell) or extracellular (outside of cells)
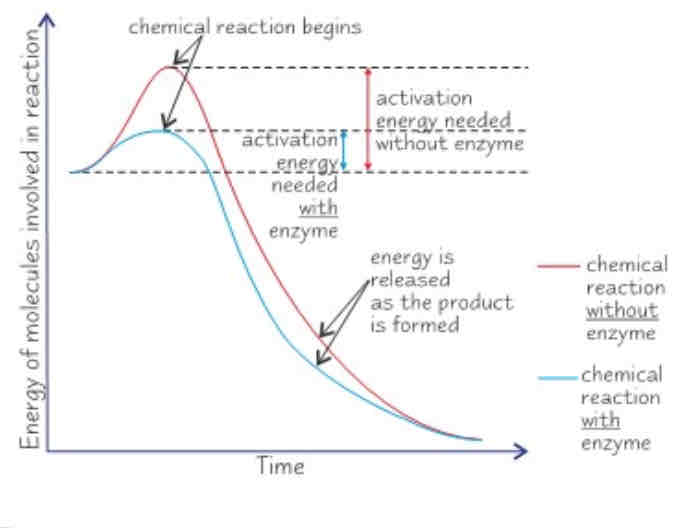
How do enzymes speed up reactions?
Enzymes lower the activation energy of a reaction, this is the certain amount of energy needed for a reaction to begin. This happens when the substrate and enzyme form the enzyme-substrate complex, for two reasons:
If two substrate molecules need to be joined, being attached to an enzyme holds them close together which reduces any repulsion and means they can bond more easily.
When the enzyme is in the active site, this puts more strain on the bonds in the substrate, allowing it to break up more easily.
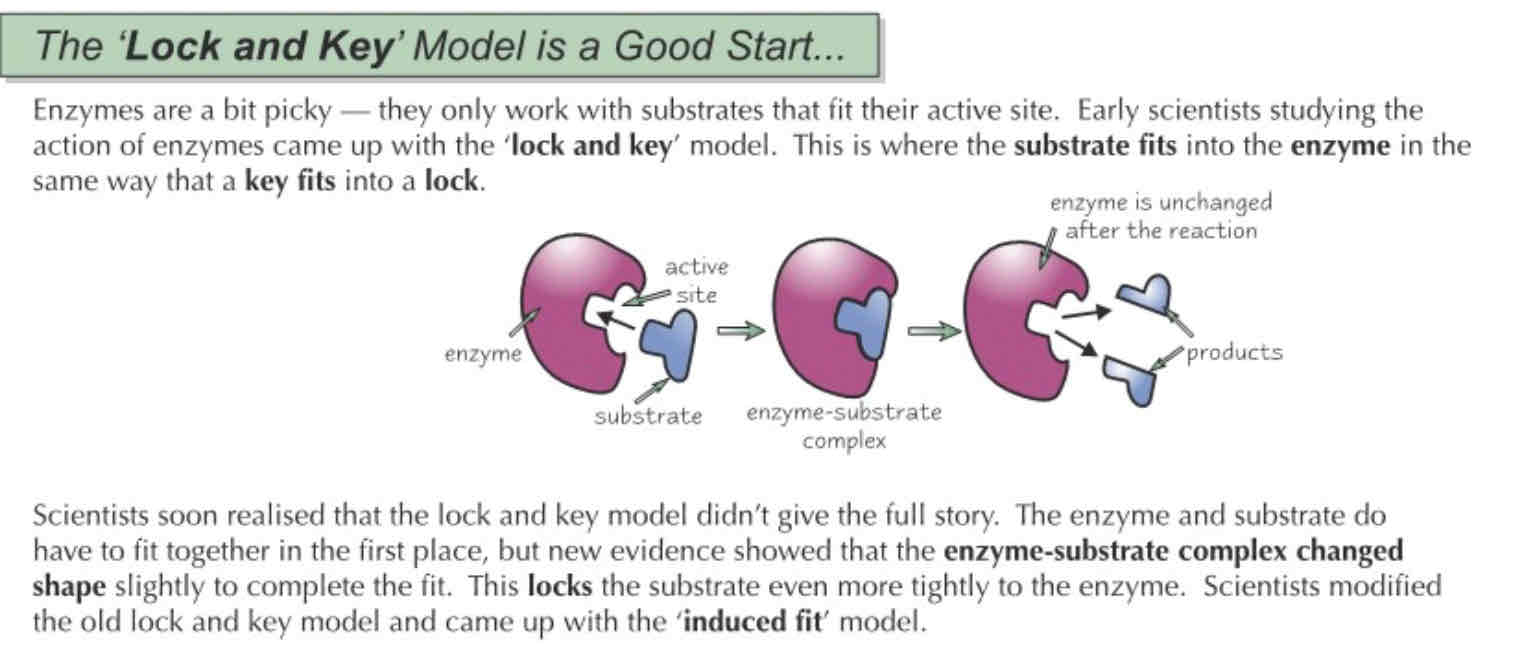
Lock and key model
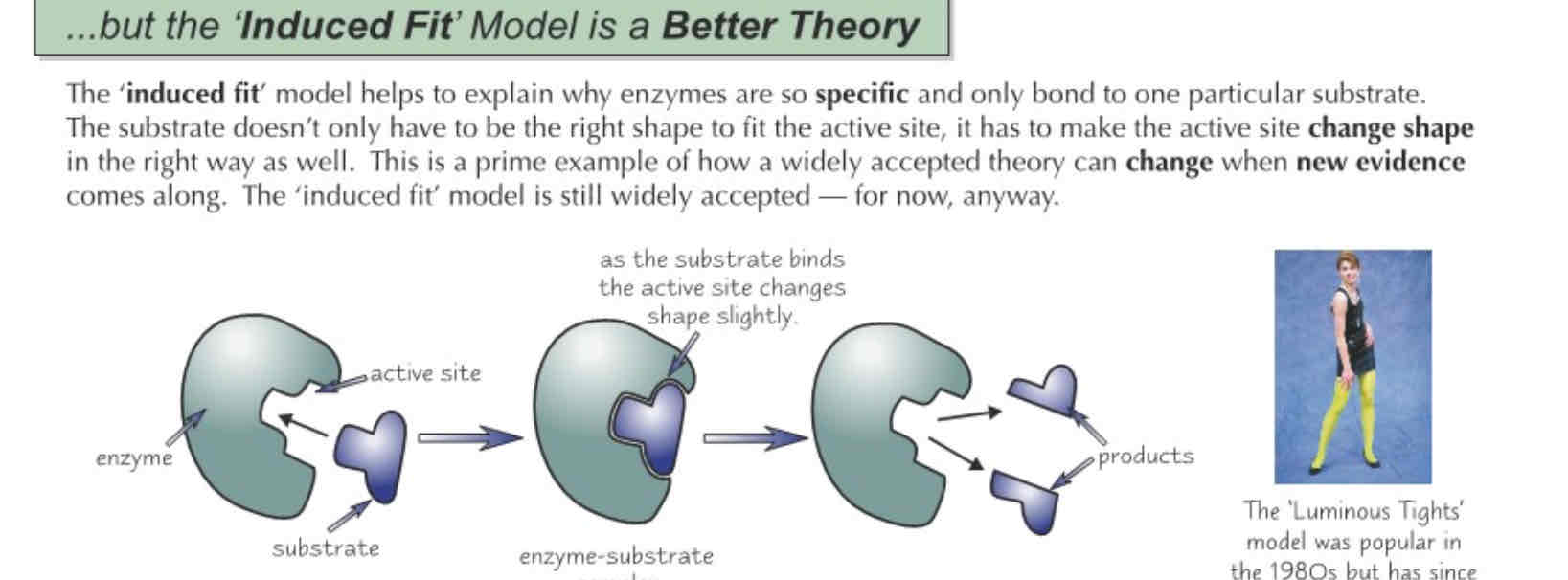
Induced fit model
Temperature on Enzyme Activity

Ph on Enzyme Actitity

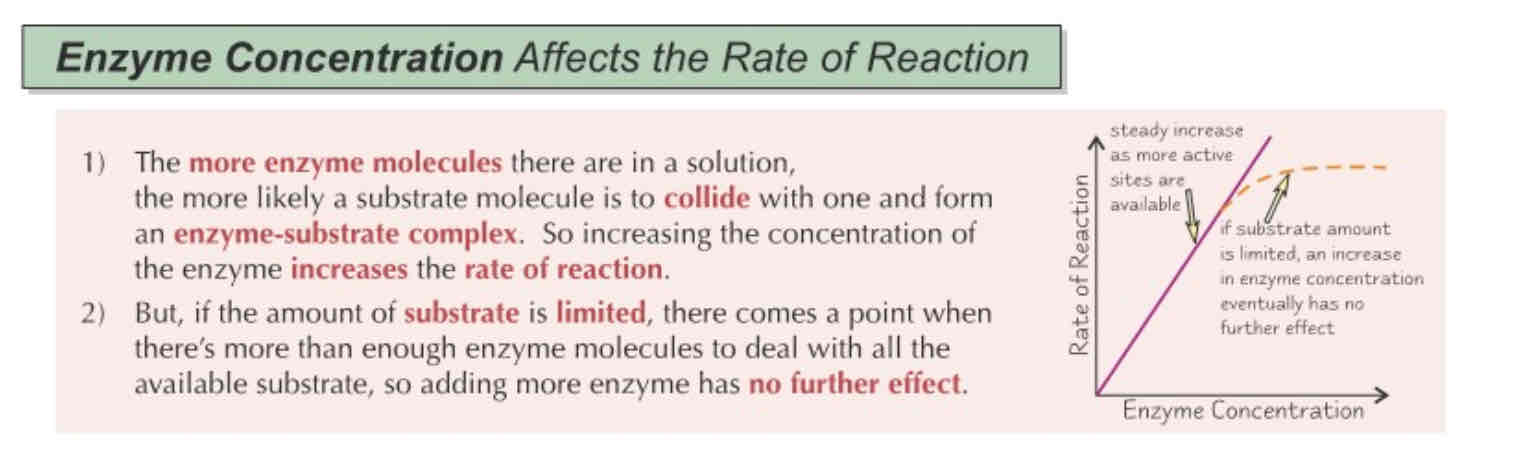
Enzyme concentration on Enzyme Activity
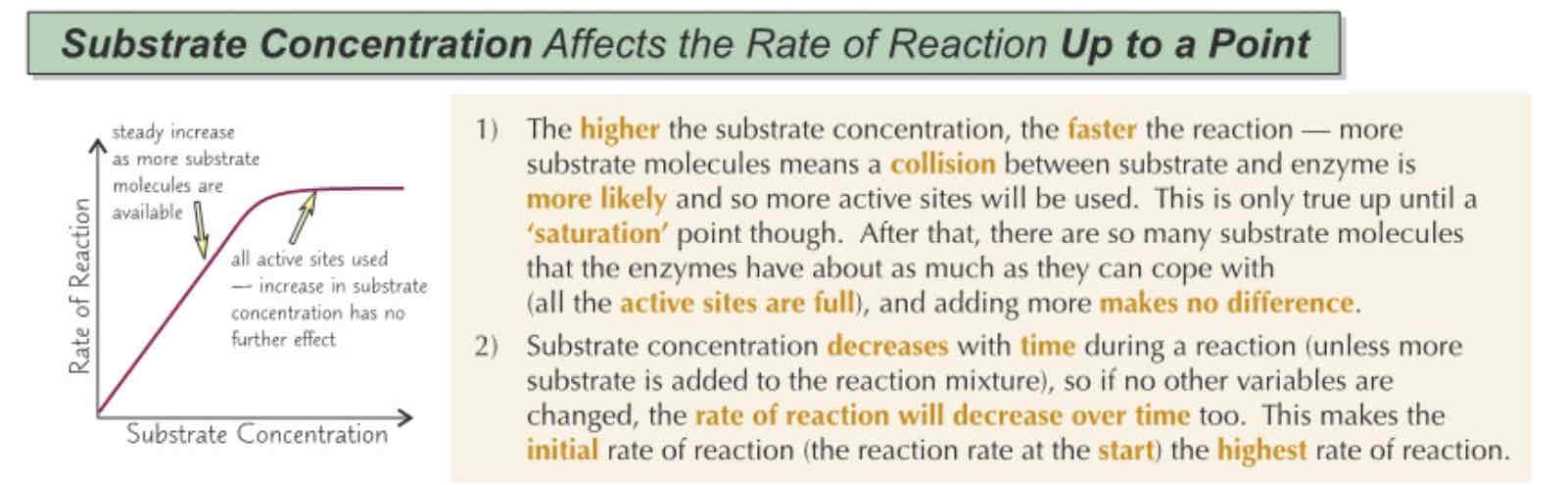
Substrate concentration on Enzyme Activity
Enzyme inhibitors
Enzyme activity can be inhibited (prevented) from occurring, using an enzyme inhibitor which are molecules that bind to the enzyme they inhibit
Competitive enzyme inhibitors
Have a similar shape to the substrate of the enzyme they’re trying to inhibit
Will compete with the substrate to bind to the enzyme’s active site first.
If they bind, they will occupy the active site which blocks other substrate from accessing it.
The extent to which an enzyme is inhibited depends on the concentration of inhibitor and substrate. For example, more inhibitors = more enzyme active sites blocked = slower rate of reaction til plateau.
Non-competitive enzyme inhibitors
These may have different shapes to the substrate as they bind to an enzyme outside of it’s active site.
The inhibitors binding to the enzyme causes the active site on the enzyme to change shape, so that the substrate can no longer bind to the enzyme.
These inhibitors don’t compete with the substrate, as they’re different shapes.
This means increasing the concentration of substrate won’t increase the rate of reaction.
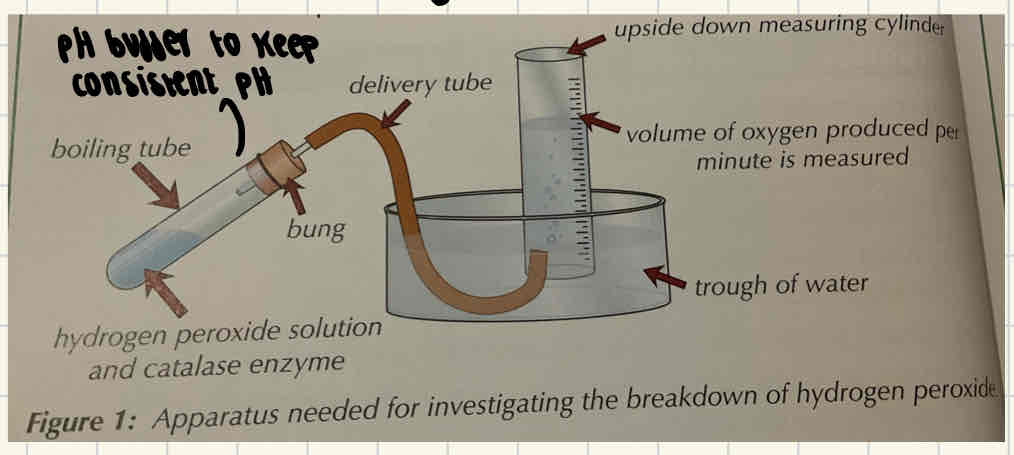
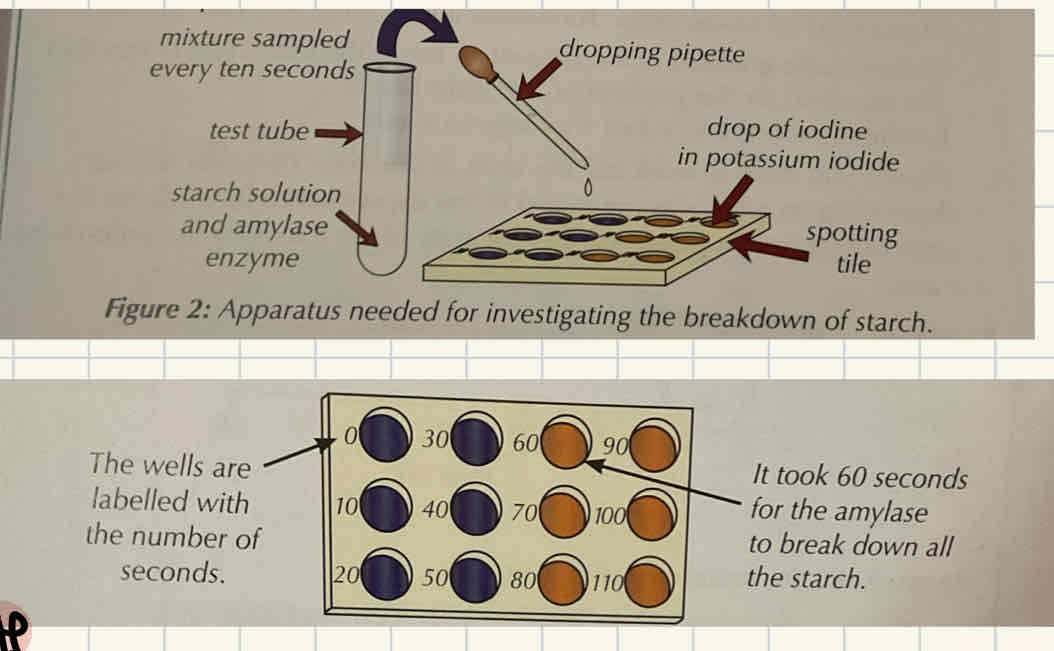
Required practical 1 - What are the two ways of measuring rate of an enzyme controlled reaction?
Measuring how fast the product of reaction appears using a water bath and a delivery tube.
Measuring how fast the substrate is broken down through using an indicator test.
Measuring rate of reaction based on how fast the product appears
Put each boiling tube into a different temperature of water bath (e.g. 10, 20, 30, 40, 50 degrees)
Use a Pipette to ad the same volume of enzyme to each boiling tube.
Connect the delivery tube to the boiling tube through the bung and guide this under water to the measuring cylinder.
Record how much oxygen (product) is produced in the first minute.
Repeat 3x to calculate a mean.
Measuring how fast the substrate is broken down using an indicator test
Drop an indicator (for example, potassium iodide) into each well and label each well per 10s
Mix concentrations of enzyme and substrate together with pH buffer and drop this in every 10 seconds into the appropriate well.
Record changes when observing the colour to see when the substrate is no longer present.
Repeat 3x to calculate mean.
Required practical 1 - Variables
Only one variable should be changed (independent variable), all others should remain the same.
Examples of variables which may be changed: pH, temperature, substrate concentration, enzyme concentration.
How to estimate initial rate of reaction
A tangent is used to estimate the initial rate of reaction. This is drawn right at the beginning of the graph when time = 0.
The gradient is then calculated through change in y/change in x.