3.3.14 Organic synthesis
1/47
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
48 Terms
AS Reactions
Free-radical substitution of alkanes
Addition reactions of alkenes
Reactions of halogenoalkanes
Oxidation of alcohols
A2 Reactions
Reduction (of alkenes, nitriles, aldehydes, ketones)
Acylation using acyl chlorides and anhydrides
Esterification and hydrolysis
Reactions of benzene
Hints and Tips
1. KNOW your organic chemistry!!!
2. Be able to link reactions together in a sequence:
i.e. alkene -> halogenoalkane -> amine -> amide
3. Remember to learn the names and formulas of reagents and give the reaction conditions.
4. Nearly all synthesis reactions includes a halogenoalkane, these are useful molecules as they can react to produce a wide range of other functional groups.
5. If the final product has the same functional group on each end of the molecule you will probably need to add Br2 to an alkene, then continue from there.
6. If the final product has one more carbon in the chain than the starting material you need to add KCN to make a nitrile then change the functional group.
There are two main types of question:
Most common: Reaction flow chart, you will be asked different questions about different reactions in the sequence. This could include names, equations, mechanisms and conditions.
Less common (section B): You may be asked how to generate a product from a given reactant in a specific number of steps.
Green Chemistry is safe, conserves raw materials and energy and is more cost effective than conventional methods.
There are three main ways to make chemical processes 'greener':
Redesign production methods to use different, less hazardous starting materials
Use milder reaction conditions, better catalysts and less hazardous solvents
Use production methods with fewer steps and higher atom economy.
Suggest why is it better to prevent pollution and the production of hazardous materials than to produce them and then clean them up?
Ideas that: not all pollution may be cleared up; energy will be wasted producing unwanted products + cleaning them up
Why might using a catalyst make a chemical process 'greener'?
Using a catalyst may mean that the process can be carried out at a lower temperature, which saves energy and lowers cost.
Suggest why scientists aim to design a process that does not require a solvent.
Solvents are usually other organic molecules that need to be evaporated off from the product which can cause harmful pollution such as greenhouse gases and contribute to global warming.
Suggest why it is better to use the fewest number of steps in a reaction sequence.
Less waste and by-products are produced with fewer steps.
Yield will also be higher as less of the product will be lost during extraction and purification after each step.
Atom Economy
Percentage atom economy
= mass of desired product/ x 100
mass of all reactants
Explain why using reactions with high atom economy is important for sustainable development.
More efficient use of resources; less waste; less pollution; less energy used.
Example: 1,2-dichloroethane
1,2-dichloroethane can be produced in two possible ways:
Reaction between ethane and chlorine, in the presence of UV light
Reaction between ethene and chlorine
Other than the lower atom economy, suggest one other reason why the reaction between ethene and chlorine is preferred in the production of 1,2-dichloroethane.
The reaction with ethene has a higher yield because further substitution can occur with ethane during free radical substitution.
There is only one possible product of the reaction with ethene, the product of free radical substitution with ethane would need to be distilled off from the other substitution products.
Reactions of alkanes
Free Radical Substitution:
- Alkane + X2 (halogen) -> halogenoalkane + HX
Reactions of alkenes
Electrophilic addition:
- Alkene + X2 -> dihalogenoalkane
- Alkene + HX -> halogenoalkane
- Alkene + H2O (+ H2SO4 catalyst) -> Alcohol
Addition:
- Alkene + H2O (+ H3PO4 catalyst) -> Alcohol
Reactions of halogenoalkanes
Nucleophilic substitution:
- Halogenoalkane + KCN (H+ catalyst) -> nitrile + KCl
- Halogenoalkane + NH3 -> Amine (+ ammonium chloride)
- Halogenoalkane + NaOH (aq) -> Alcohol + NaCl
Elimination:
- Halogenoalkane + NaOH (ethanolic) -> Alkene + H2O + NaCl
Reactions of alcohols
Oxidation:
- 1o alcohol + [O] -> aldehyde + H2O
- 1o alcohol + 2[O] -> carboxylic acid + H2O
- 2o alcohol + [O] -> ketone + H2O
Dehydration:
- Alcohol -> Alkene + H2O
Nucleophilic addition-elimination:
- Alcohol + acyl chloride -> ester + HCl
Reactions of aldehydes/ketones
Oxidation:
- Aldehyde + [O] -> carboxylic acid
Nucleophilic addition / reduction:
- Aldehyde/ketone + [H] -> 1o/2o alcohol
Nucleophilic addition / reduction:
- Aldehyde/ketone + KCN (H+ catalyst) -> hydroxynitrile
Reactions of carboxylic acids
Esterification:
- Carboxylic acid + alcohol ⇌ ester + water
Reactions of nitriles
Reduction:
- Nitrile + [H] -> amine
Reactions of amines
Further nucleophilic substitiution:
- Amine + haloalkane -> 2o/3o amine or 4o ammonium salt
Nucleophilic addition-elimination:
- Amine + acyl chloride -> N-substituted amide + HCl
Reactions of acyl chlorides (acid anhydride forms carboxylic acid)
Nucleophilic addition-elimination:
- Acyl chloride + water -> carboxylic acid + HCl
- Acyl chloride + alcohol -> ester + HCl
- Acyl chloride + ammonia -> amide + HCl
- Acyl chloride + amine->N-substituted amide + HCl
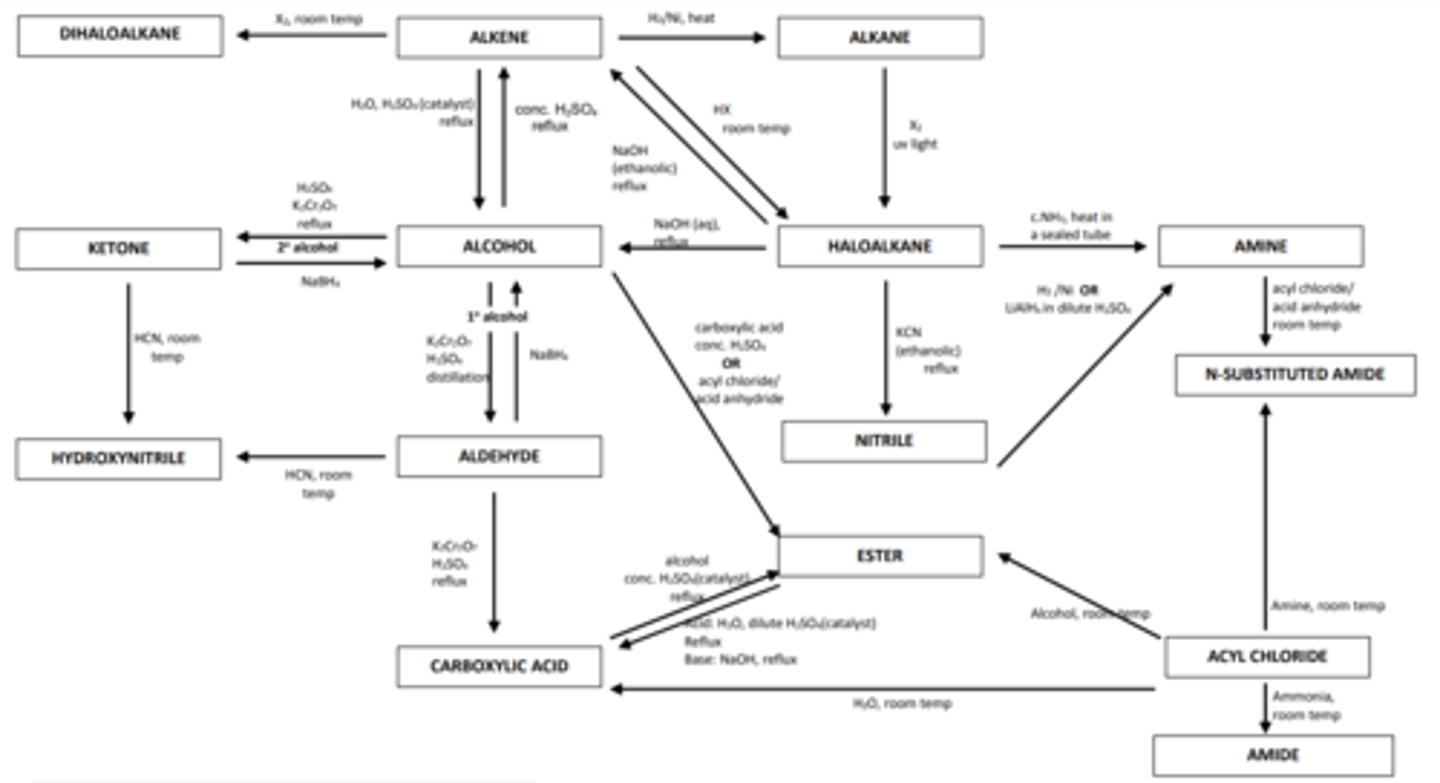
Aliphatic Synthesis
Aromatic Synthesis
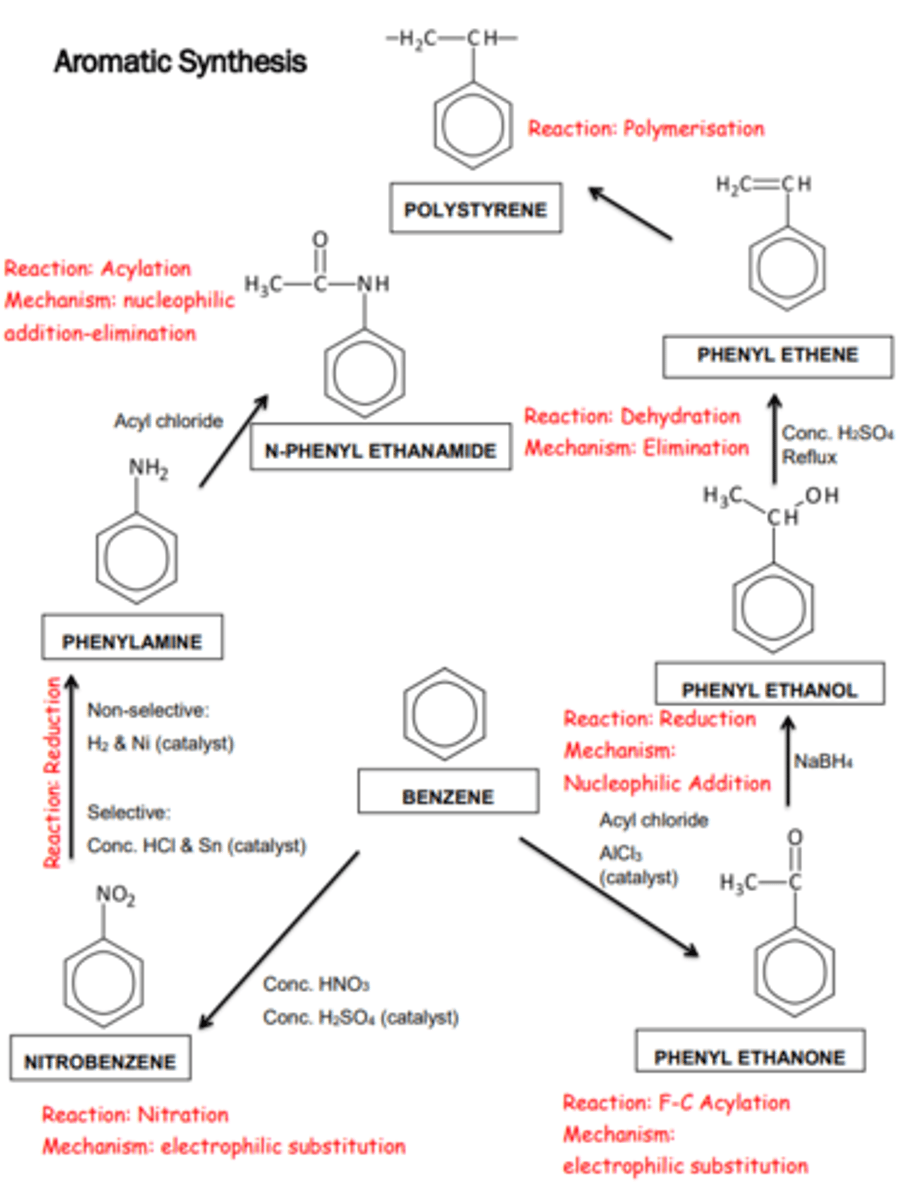
FC Acylation: C6H6 + CH3COCl -> C6H5COCH3 + HCl
Reduction: C6H5COCH3 + 2[H] -> C6H5CH(OH)CH3
Dehydration: C6H5CH(OH)CH3->C6H5CH=CH2 + H2O
Nitration: C6H6 + HNO3 -> C6H5NO2 + H2O
Reduction: C6H5NO2 + 6[H] -> C6H5NH2 + 2H2O
Acylation:
C6H5NH2+CH3COCl->C6H5NHCOCH3 + HCl
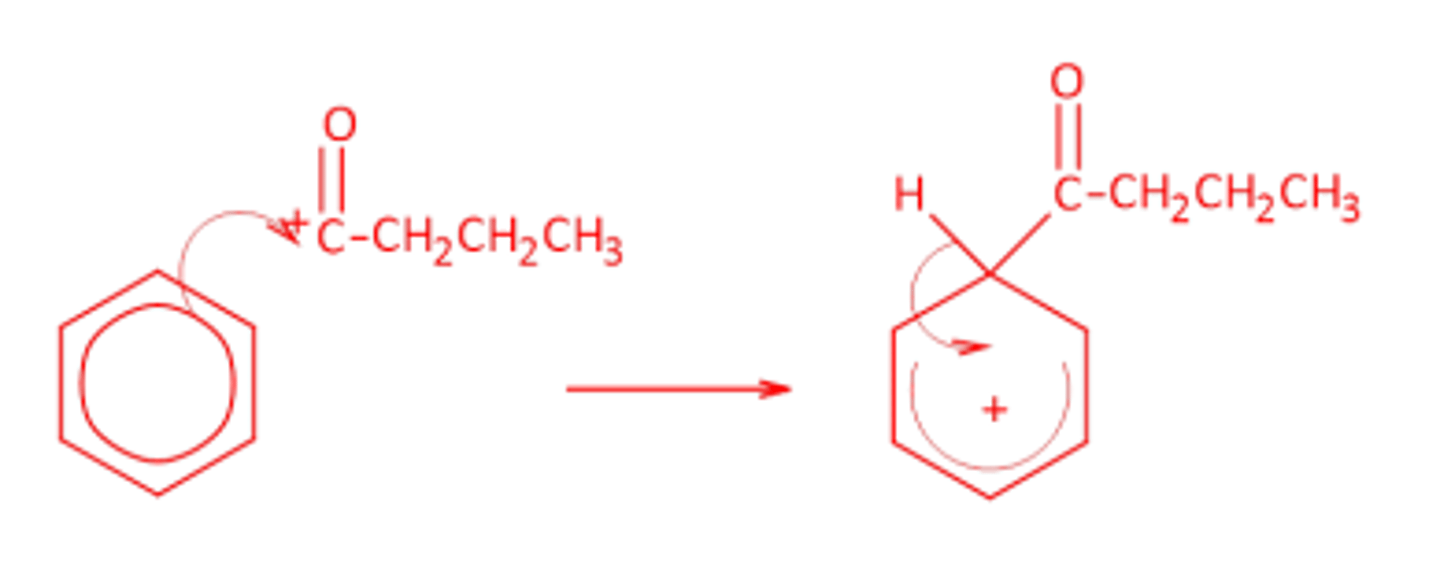
Draw the mechanism for the production of phenylbutanone, write equations to show that AlCl3 acts as a catalyst in the reaction.
Electrophile:
CH3CH2CH2COCl + AlCl3 -> CH3CH2CH2C+O + AlCl4-
Catalyst regeneration:
AlCl4- + H+ -> AlCl3 + HCl
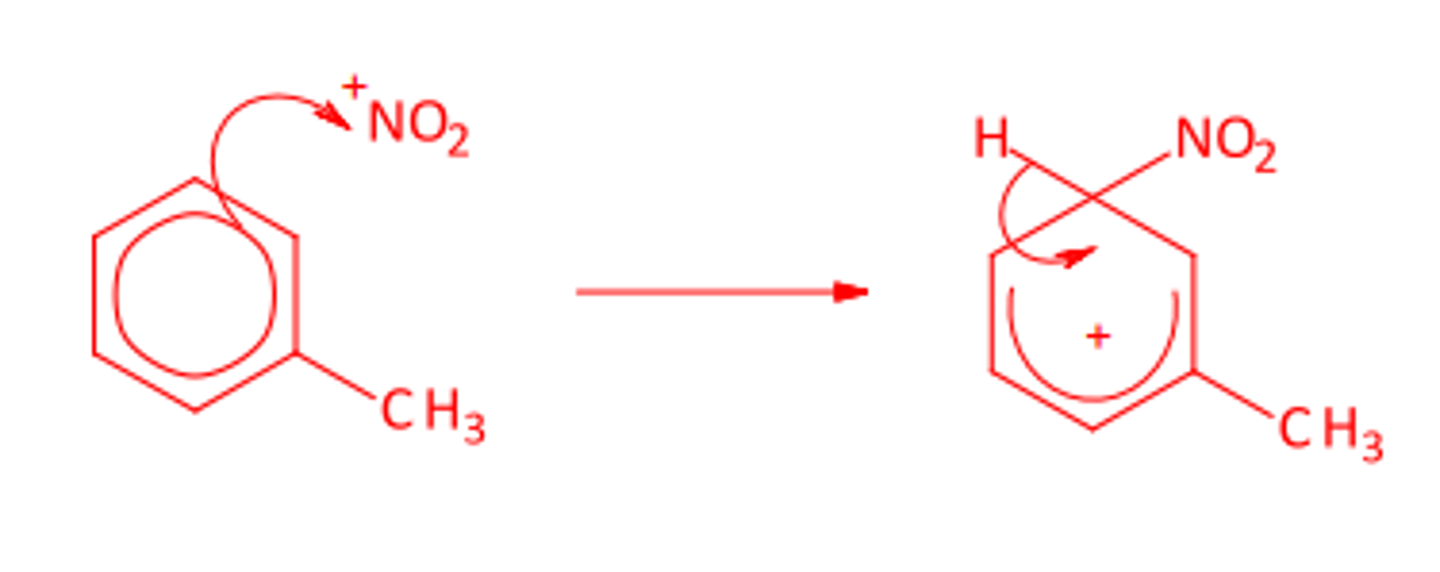
Write the equation for the production of the +NO2 electrophile and draw the mechanism for the production of 3-nitromethylbenzene.
Electrophile:
HNO3 + 2H2SO4 -> +NO2 + H3O+ + 2HSO4-
Functional group: Alkene
Reagent(s): Bromine water
Observations: Orange solution turns colourless
Functional group: Halogenoalkane
Reagent(s): Silver nitrate
Observations:
White ppt = chloro, dissolves in d. NH3
cream ppt = bromo, dissolves in c. NH3
yellow ppt = iodo, does not dissolve
Functional group: 1o/ 2o Alcohols
Reagent(s): Acidified potassium dichromate
Observations: Orange solution turns green
Functional group: Aldehydes
Reagent(s): Tollens reagent / Fehlings solution
Observations:
T: silver mirror (ppt) forms
F: blue solution produces a red-brick ppt
Functional group: Carboxylic acids
Reagent(s): Sodium (hydrogen) carbonate
Observations:
Effervescence/ bubbles of CO2 gas evolved
Functional group: Acyl chlorides
Reagent(s):
Water / ethanol /ammonia
OR
AgNO3
Observations:
White misty fumes produced
OR
White ppt that dissolves in dilute NH3
Functional group: Amine
Reagent(s): Universal indicator
Observations: Green indicator turns blue
Propylamine can be produced in two possible ways:
1-step reaction between 1-chloropropane and ammonia
2-step reaction between 1-chloroethane and HCN, then reduction with hydrogen
Suggest why the 2-step reaction is preferred during industrial production of propylamine.
The two step reaction has a higher yield.
There are further substitution products with the one step reaction, propylamine would need to be separated from the other products.
Aliphatic Synthesis Pathways:
The aliphatic synthesis chart looks complicated but the main processes usually involve a halogenoalkane so start from there.
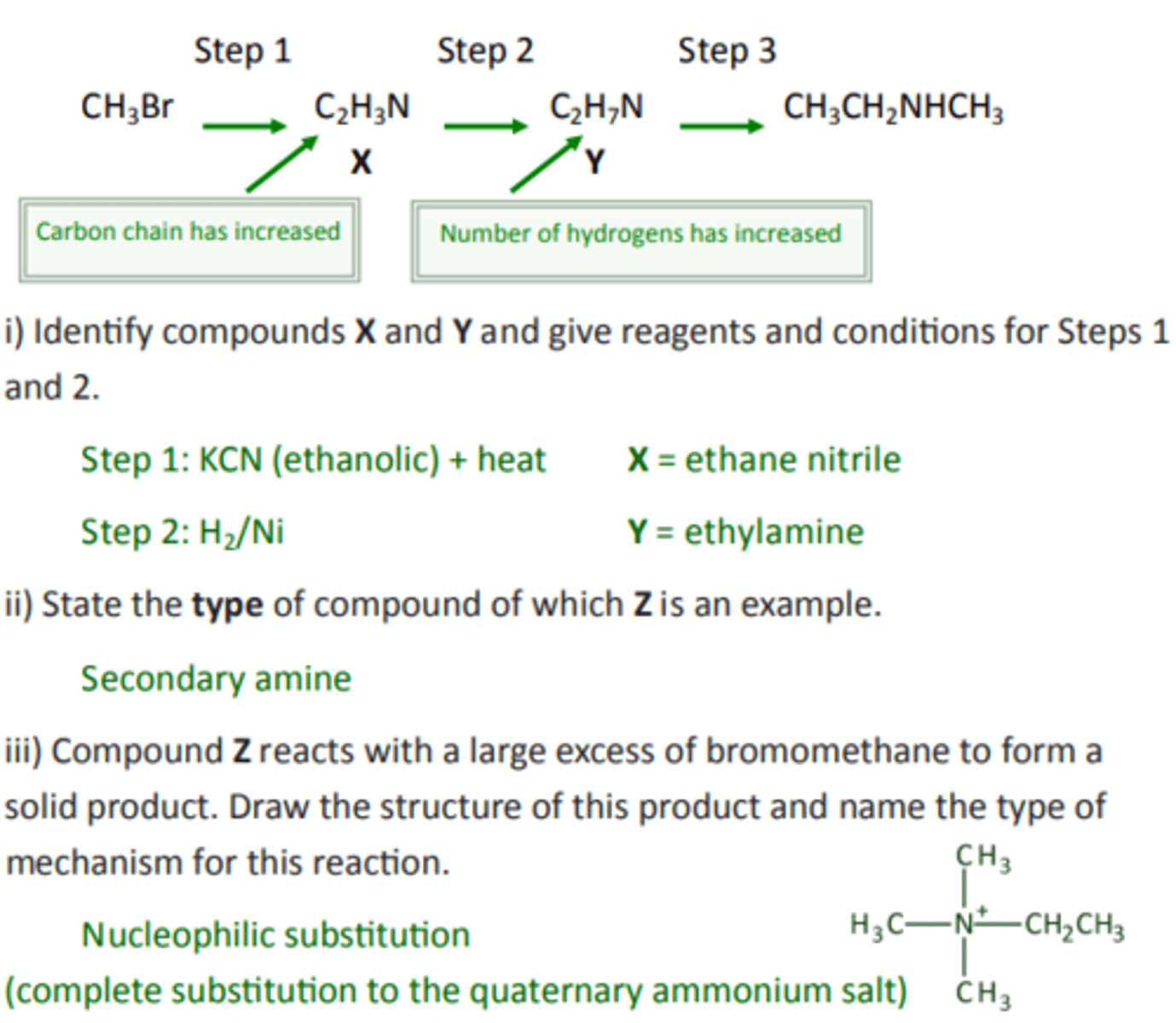
Example: Compound Z can be formed via compounds X and Y in the three step synthesis shown below:
i) Identify compounds X and Y and give reagents and conditions for Steps 1 and 2.
ii) State the type of compound of which Z is an example.
iii) Compound Z reacts with a large excess of bromomethane to form a solid product. Draw the structure of this product and name the type of
mechanism for this reaction.
i) Step 1: KCN (ethanolic) + heat X = ethane nitrile
Step 2: H2/Ni Y = ethylamine
ii) Secondary amine
iii) Nucleophilic substitution
(complete substitution to the quaternary ammonium salt)
Aromatic Synthesis Pathways:
Initially start with a reaction on the benzene ring, this substituent then further reacts to continue the synthesis.
Alternative questions ask you to plan a synthesis in a number of steps.
Give the reagent and conditions for the synthesis of N-phenylethanamide from benzene, identify any intermediate products.
Step 1: Benzene + c.HNO3 (+.c.H2SO4) to produce nitrobenzene.
Step 2: Reduction of nitrobenzene using c.HCl + Sn to form phenylamine.
Step 3: Nucleophilic addition-elimination using ethanoyl chloride to form N-phenylethanamide.
Important Synthesis Steps:
Synthesis questions involve 3 multilinked reactions, important steps are:
Halogenoalkanes: can form multiple products (nuc sub and elimination).
Nitriles: increases the carbon chain length.
Alkene + Br2: adds 2 functional groups to the molecule.
Green Chemistry:
Green chemistry involves using the process with the least number of steps or the highest atom economy.
It is better to prevent pollution than to clean it up because not all pollution can be removed/ cleaned.
Sometimes an indirect method is used rather than a direct method if further substitution could occur in the direct method.

Functional Group Tests:
Reagents and observations are needed. The reagent must be correct to gain credit for the observation. If the formula of the reagent is given then it has to be chemically correct.
Often the molecules are drawn at odd angles or backwards to make you think more about which functional group is shown in each molecule.
Observations should include the colour and state of the reagent and the change in the colour/state.
Alkene:
(Reagent) Add bromine water.
(Observation) Bromine water decolourises.
Alcohols:
(Reagent) Add potassium dichromate (K2Cr2O7), acidified with sulphuric acid (H2SO4) and heat. (Observations) 1o & 2o alcohols: orange
solution to blue solution.
3o alcohols: NVC (no visible change).
Aldehydes:
(Reagent):
Add Tollen's reagent ([Ag(NH3)2]+) and heat.
(Observation) silver mirror/ppt forms.
Alternative:
(Reagent) Add Fehling's solution and heat. (Observation) Blue solution forms a red-brick ppt.
Carboxylic acids:
(Reagent):
Add sodium carbonate solution (Na2CO3).
(Observation) effervescence.
Acyl chlorides:
(Reagent):
Add water/ethanol/ammonia/methylamine
(any NAMED alcohol or amine).
(Observation) White misty fumes.
Halogenoalkanes:
(Reagent) Silver nitrate solution and heat.
(Observation) coloured ppt produced.
White ppt = chloroalkane (ppt dissolves in dilute NH3).
Cream ppt = bromoalkane (ppt dissolves in conc
NH3).
Yellow ppt = iodoalkane (ppt does not dissolve in NH3).
Amines:
(Reagent) Add universal indicator.
(Observation) Indicator turns blue.
Alternative:
Use a pH meter, reading between pH 10-11.
No chemical test for ketones, acid anhydrides, amides or esters:
these will always be NCV (never write nothing).