Aromaticity
1/86
Earn XP
Description and Tags
Short Revision for Aromaticity, suitable for JEE and IAT! Answer mode: Answer with Definition. Question mode: just flashcards. I'll add more and make it more comprehensive in the future maybe!
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
87 Terms
What is the general formula for aromatic compounds?
C_nH_{2n-6y}
where:
n is the number of carbons
y is the number of rings
What does naphthalene look like?
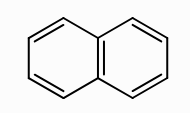
What is Huckel’s rule?
if a compound has 4n+2 \pi electrons (where n is whole number), the compound is aromatic.
if a compound has 4n \pi electrons (where n is whole number), the compound is anti-aromatic.
if the number of \pi electrons does not fit those two rules, the compound is nonaromatic.
What should be the hybridisation of carbons in aromatic and antiaromatic compounds?
sp²
it can be sp in very big rings
What is the stability order of aromatic, nonaromatic, and anti-aromatic compounds?
aromatic > nonaromatic > anti-aromatic
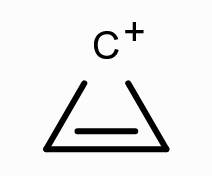
What is the aromaticity of this compound?
aromatic
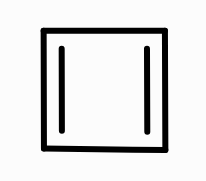
What is the aromaticity of this compound?
anti-aromatic
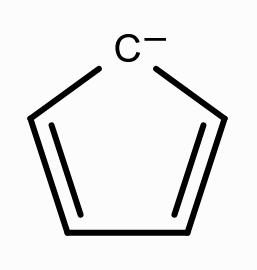
What is the aromaticity of this compound?
aromatic
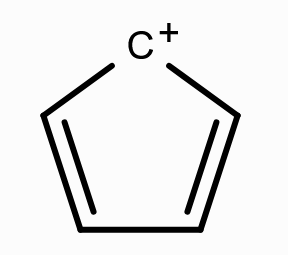
What is the aromaticity of this compound?
anti-aromatic

What is the aromaticity of this compound?
non-aromatic
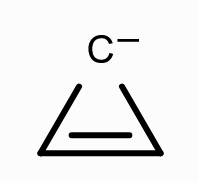
What is the aromaticity of this compound?
anti-aromatic
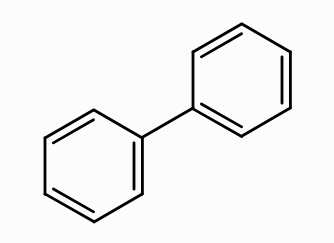
What is the aromaticity of this compound?
aromatic
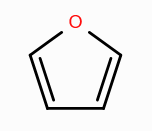
What is the aromaticity of this compound?
aromatic
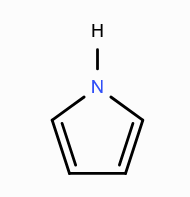
What is the aromaticity of this compound?
aromatic

What is the aromaticity of this compound?
aromatic
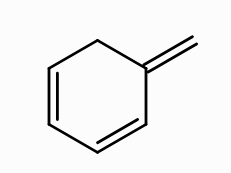
What is the aromaticity of this compound?
nonaromatic
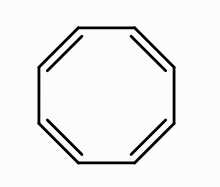
What is the aromaticity of this compound?
it’s nonaromatic because it’s not planar
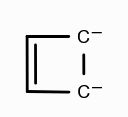
What is the aromaticity of this compound?
aromatic
What is the aromaticity of this compound?
aromatic
When preparing arenes from alkanes, what reagent do you use? (there are 2 choices)
Cr_2O_3 or Al_2O_3 and heat
What are the byproducts when forming arenes from alkanes?
4H_2
What is the reagent when forming arenes from alkynes?
red hot iron tube
What is the reagent when forming arenes from phenol?
Zn and heat
(it is a reduction reaction)
What is the reagent when forming arenes when there is an aldehyde present?
(i) NH_2-NH_2
(ii) OH^-/\Delta
What is the reagent when forming arenes when there is a ketone present?
Zn-Hg and HCl
What is the reagent when forming arenes from benzoic acid?
Soda Lime! (NaOH+CaO) and heat
What is the byproduct formed when forming arenes from benzoic acid?
CO_2
What is the reagent when forming arenes from sulphonic acid?
H_3O^+/\Delta
yes it is hydrolysis
What is the reagent when forming arenes from a diazonium salt?
H_3PO_2/C_2H_5OH
What is the reagent when forming arenes from Grignard’s Reagent?
H_2O or C_2H_5OH
What is the byproduct when forming arenes from Grignard’s Reagent
Mg(OH)Br
How do you form arenes from Wurtz’s fitting reaction?
You take two chlorobenzenes and react them with Na/\text{dry ether} and heat to form this thang
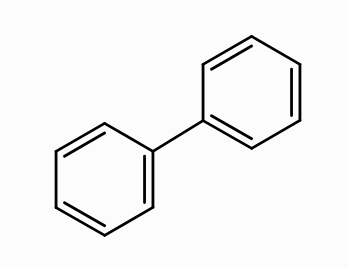
What is the substrate when forming arene from Wurtz’s fitting reaction?
chlorobenzene
What is the reagent when forming arene from Wurtz’s fitting reaction?
Na/\text{dry ether}
What is the characteristic reaction of benzene, or benzene derivatives?
Electrophilic Substitution Reaction
What is the mechanism of Electrophilic Substitution Reactions for arenes?
An electrophile attacks one of the carbons on benzene, which breaks one of its pi bonds. This leaves a positive charge on one of the adjacent carbons, which resonates through ortho and para positions. This intermediate has lost its aromaticity. It’s called arenium ion.
A nucleophile comes along and abstracts the hydrogen proton that is attached to the same carbon as the electrophile, leaving the extra electron to form a bond with the adjacent carbon with positive charge, thus gaining back aromaticity.
How does the electron density on the benzene ring affect the reactivity of Electrophilic Substitution Reactions on the benzene ring?
Reactivity increases with increase in electron density.
How does the stability of the Arenium ion affect the rate of the Electrophilic Substitution Reaction?
the stability of the Arenium ion increases the rate of reaction
How does the electron-donating-nature of the electron donating group affect the rate of Electrophilic Substitution Reaction?
the more the electron-donating-nature, the greater the rate of the reaction.
What does the Potential Energy graph look like for Electrophilic Substitution Reactions of Arenes?

What is the characteristic of Ortho/Para directing groups on Arenes?
they provide +M/+I/+H effects
They increase the electron density on benzene ring at ortho and para positions.
Is -NH_2 an activating group or not?
yeah
Is -NHR an activating group or not?
yeah
Is -NR_2 an activating group or not?
yeah
Is -OH an activating group or not?
yeah
Is -OR an activating group or not?
yes
Is -NHCOR an activating group or not?
yes
Is -SH an activating group or not?
yes
Is -OCOR an activating group or not?
yes
Is -CH_3 an activating group or not?
yes
What is the characteristic of Meta directing groups on Arenes?
they provide -M/-H/-I effect
they decrease the electron density at ortho and para positions on benzene ring
Are +M groups on benzene activating or deactivating groups?
activating
Are -M groups on benzene activating or deactivating groups?
deactivating
Is -CHO an activating group or not?
nope
Is -COOH an activating group or not?
nope
Is -COOR an activating group or not?
nope
Is -COR an activating group or not?
nope
Is -CN an activating group or not?
nope
Is -NO_2 an activating group or not?
nope
Is -SO_3H an activating group or not?
nope
Is -CCl_3 an activating group or not?
nope
Why is FeCl_3 a Lewis Acid?
Because Fe accepts a Lone Pair into its vacant d-orbital.
Why is AlCl_3 a Lewis Acid?
Because Al has an incomplete octet and can accept 2 more electrons.
If benzene and chlorobenzene are present in a solution together, what would an electrophile prefer to react with?
benzene.
-I of chlorobenzene wins over +M
Is -Cl ortho/para directing or meta directing?
Ortho/Para directing
+M wins over -I.
In Halogenation of benzene, what is the reagent?
Cl_2 or Br_2 with FeCl_3
In Halogenation of benzene, what could be the byproducts?
HCl/HBr
In Halogenation of Toluene, if you want to add the halogen to the benzene ring, what reagent do you use?
Cl_2/Br_2 with FeCl_3/FeBr_3
In Halogenation of Toluene, if you want to add the halogen to the alkyl group, what reagent do you use?
Cl_2/Br_2 with sunlight (h\nu)
In Nitration of toluene, what reagent do you use?
concentrated HNO_3\ + concentrated H_2SO_4
In Nitration of toluene, what is the electrophile?
NO_2^+
In Sulphonation of Toluene, what reagent do you use?
concentrated H_2SO_4
In Sulphonation of Toluene, what is the electrophile?
HS^+O_3
What is alkylation of benzene?
substitution of alkyl group (R) to benzene ring
What is acylation benzene?
substitution of acyl group (COR) to benzene ring
What is acetylation benzene?
substitution of acetyl group (COCH_3) to benzene ring
During Alkylation of benzene, what are the possible reagents used? (three of them)
R-Cl and a Lewis acid
R-OH and H^+
alkene and either H^+or a Lewis Acid
Lewis Acids can be FeCl_3/AlCl_3/ZnCl_2
What is the electrophile in Alkylation of arenes?
R^+
Why can’t you use hydrous AlCl_3 during Alkylation of arenes?
the Al atom can accept lone pair from the oxygen from water as well, leading to no formation of R^+.
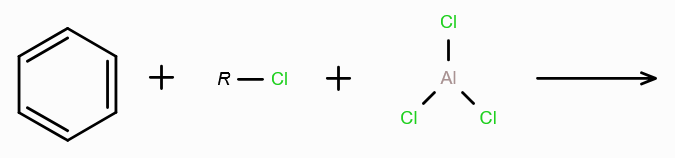
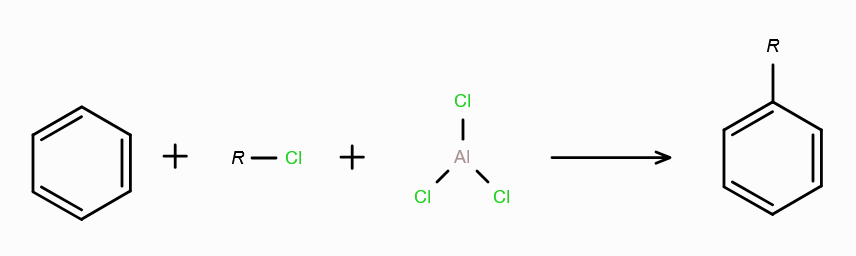
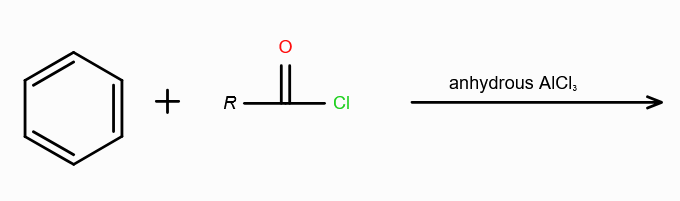
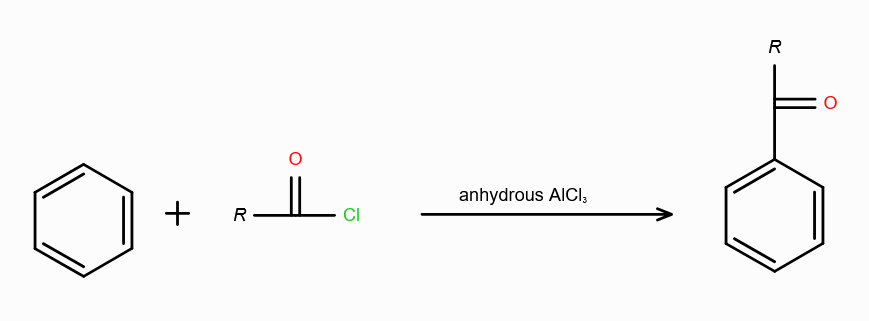
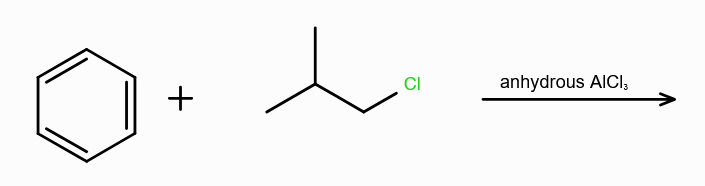
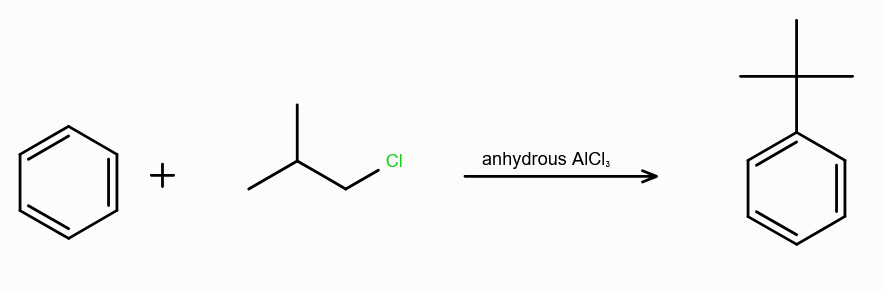
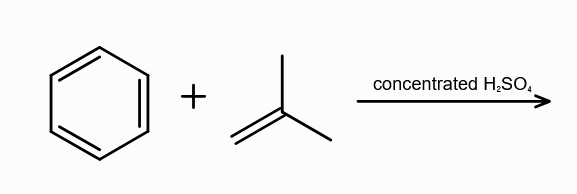
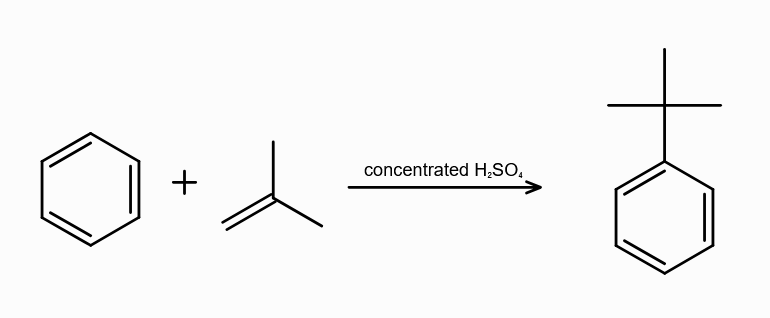
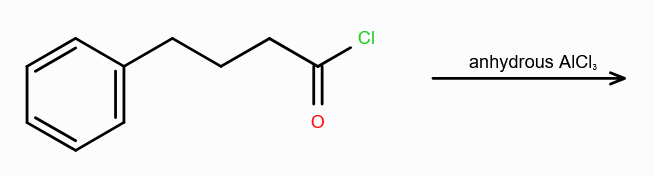
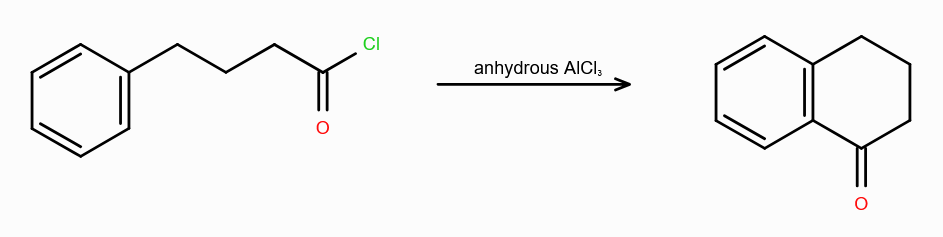
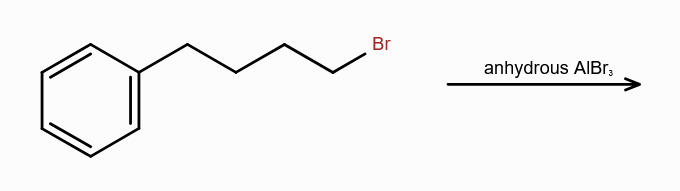
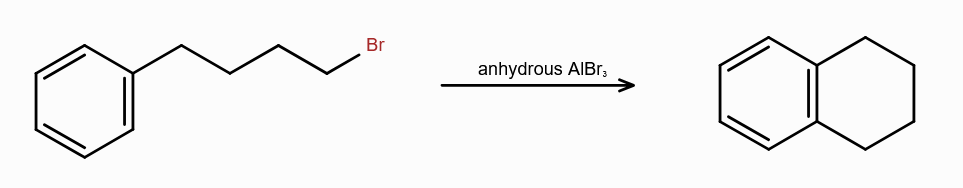
What is Friedel-Craft Reaction?
Alkylation or Acylation of Benzene and Benzene Derivatives
In Acylation of Benzene, what are the possible reagents? (2 possible)
Acid Halide (RCOX) and a Lewis Acid
Acid Anhydride (RCOCR) and a Lewis Acid
Lewis Acids can be FeCl_3/AlCl_3/ZnCl_2