Chemistry Unit 1: Atomic Theory, Kinetic Theory, Emission Spectra
1/21
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
22 Terms
atoms
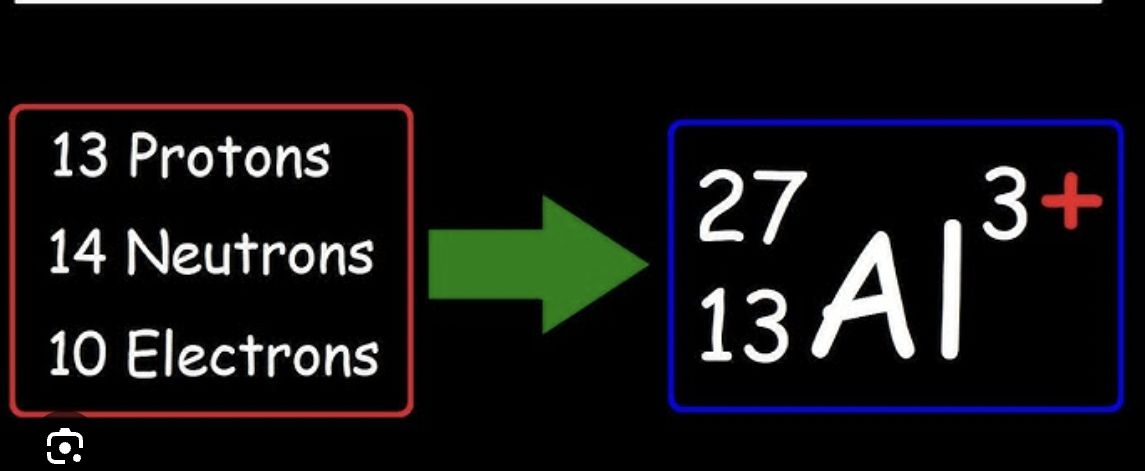
the smallest particle of a chemical element that can exist, it contains protons, neutrons and electrons. Protons + neutrons are equal to the atomic number of the element, the number below it is the amount of electrons
molecules
two or more atoms connected by chemical bonds
compounds
a substance made from two or more different elements that have been chemically joined.
mixtures
a combination of two or more substances in any proportion. This is different from a compound, which consists of substances in fixed proportions. The substances in a mixture also do not combine chemically to form a new substance, as they do in a compound.
filtration
the process in which solid particles in a liquid or gaseous fluid are removed by the use of a filter medium that permits the fluid to pass through but retains the solid particles.
solvation
Solvation is the process by which solvent molecules surround and interact with solute ions or molecules.
An important specific example of solvation is hydration, where the solvent is water.
In general, the rule of like-attracts-like applies to solvation:
Polar solutes such as sodium chloride are solvated by polar solvents such as water (polar solvated by polar)
Non-polar solutes such as icosane are solvated by non-polar solvents such as benzene; (non-polar solvated by non-polar)
Vacuum filtration
a procedure in which a pressure differential is maintained across the filter medium by evacuating the air below the filter paper. Vacuum filtration provides a force on the solution in addition to that of gravity and increases the rate of filtration. (Buchner Funnel)
Crystallisation
is a technique used for the purification of substances. A separation technique to separate solids from a solution.
Recrystallisation
also known as fractional crystallization, is a procedure for purifying an impure compound in a solvent. The method of purification is based on the principle that the solubility of most solids increases with increased temperature. This means that as temperature increases, the amount of solute that can be dissolved in a solvent increases.
Isotopes
Isotopes are atoms of the same element with different numbers of neutrons, therefore same atomic number but differing atomic mass.
Properties:
Chemically identical to each other.
physical properties vary as the weight of the particles changes
SOME isotopes have unstable nuclei, and will
undergo nuclear decay to form a more stable
nucleus (radioactive decay)
Electron configuration
the arrangement of electrons in orbitals around an atomic nucleus
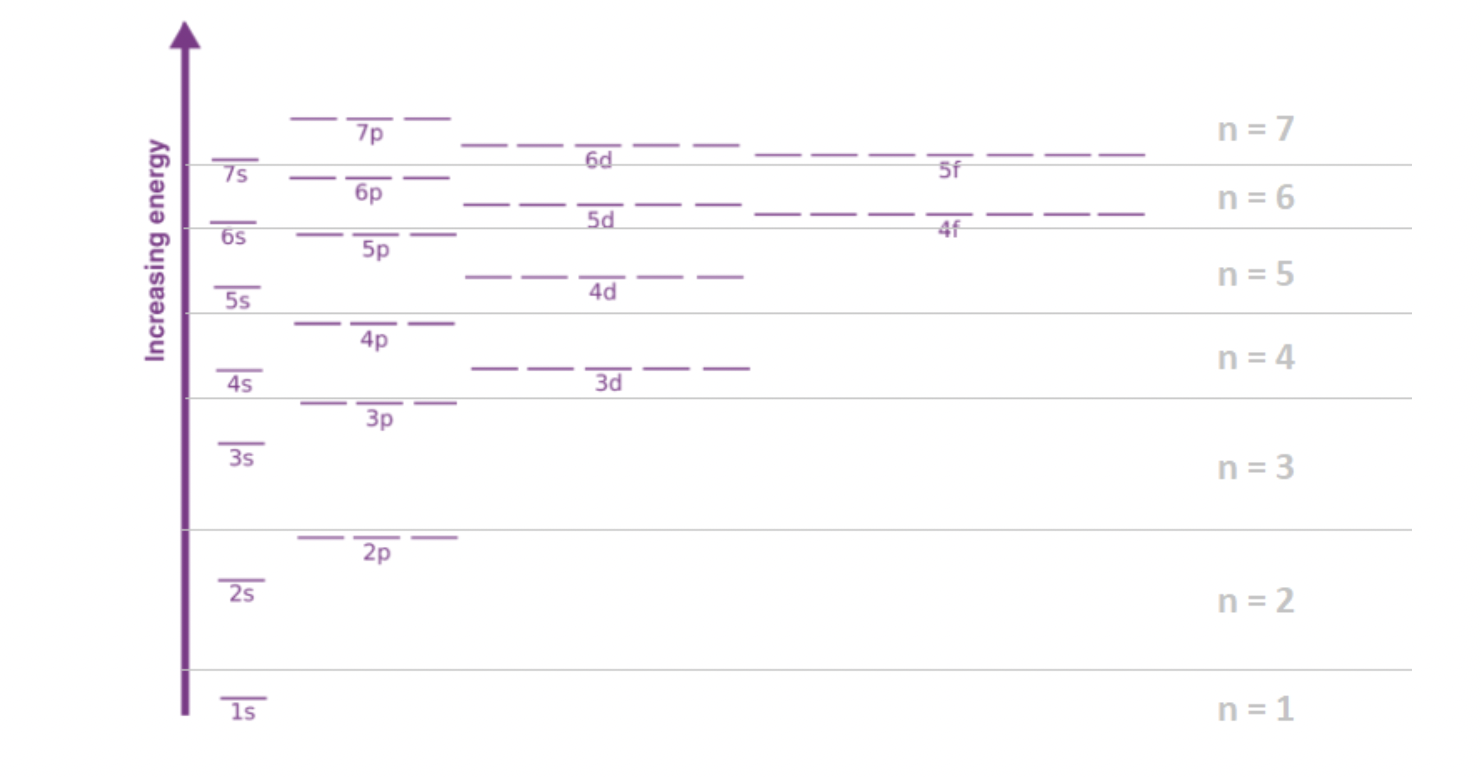
Shell n=1
2 electrons in ‘1s’, 2 electrons in total
Shell n=2
first shell filled, 2 electrons in ‘2s’, 6 electrons in ‘2p’, 10 electrons in total
Shell n=3
first and second shell filled, 2 electrons in ‘3s’, 6 electrons in ‘3p’, 10 electrons in ‘3d’, in total 18 electrons
Shell n=4
first, second and third shells filled, 2 electrons in ‘4s’, 6 electrons in ‘4p’, 10 electrons in ‘4d’, 14 electrons in ‘4f’, in total 32 electrons
3 laws for filling orbitals
the electron will always fills out the lowest available orbital first (Aufban Principal)
a pair of electrons in the same orbital can’t have the same spin (Pauli’s Exclusion Principal)
Electrons will not double up if there is an empty
orbital available at the same energy level. (Hund’s rule)
Emission spectra (ES)
the spectrum of frequencies of electromagnetic radiation emitted due to an atom or molecule transitioning from a high energy state to a lower energy state.
Emission Spectometry
The energy of light is directly proportional to frequency, and inversely proportional to wavelength.
the emission spectrum order (longer wavelength/lower frequency+energy - shorter wavelength/higher frequency+energy)
radio, microwave, infrared, visible, ultraviolet, X-ray, gamma ray
frequency (in the ES)
number of waves passing per second, inversely proportional to wavelength. c=λv (where c is the speed of light, λ is wavelength and v is frequency)
wavelength (in the ES)
distance between two wave peaks, inversely proportional to frequency. c=λv (where c is the speed of light, λ is wavelength and v is frequency)
Relations of Electrons and Photons
When the electron is gained - absorption of the exact amount of ΔE (the energy difference between the energy levels) that is neccessary to move up the energy level, when the electron is lost - release that same amount of energy in the form of a photon with exactly the amount of energy as ΔE