Covalent Compounds, Formulas, and Structures
- 4.1 Covalent Molecules
Covalent molecules are chemical compounds that are formed by the sharing of electrons between two or more atoms. These molecules are also known as molecular compounds or covalent compounds.
In a covalent bond, two atoms share one or more pairs of electrons in order to achieve a stable electron configuration. This type of bond is typically found between nonmetal atoms, as they have a high electronegativity and a tendency to gain or share electrons.
Examples of covalent molecules include ==water (H2O), carbon dioxide (CO2), methane (CH4), and ammonia (NH3).== These molecules have specific properties, such as boiling and melting points, that are determined by the strength of the covalent bonds between their atoms.
Covalent molecules can exist as gases, liquids, or solids at room temperature, depending on the strength of their intermolecular forces. They are typically not soluble in water, which is a polar solvent that interacts more strongly with ionic compounds. However, some covalent molecules can dissolve in nonpolar solvents, such as oil or gasoline.
- 4.2 Lewis Structures of Molecules
Lewis structures are diagrams that show the arrangement of atoms and electrons in a molecule. They are named after Gilbert ==N.== Lewis, who first introduced the concept in 1916.
In a Lewis structure, each atom is represented by its chemical symbol, and valence electrons are represented by dots placed around the symbol. For example, in the Lewis structure for water ==(H2O),== the oxygen atom is represented by an =="O"== and has two pairs of valence electrons represented by four dots around it, while each hydrogen atom is represented by an =="H"== and has one valence electron represented by a single dot.
To draw a Lewis structure, you should follow these steps:
- Count the total number of valence electrons in the molecule by adding the number of valence electrons for each atom.
- Determine the central atom, which is usually the atom with the lowest electronegativity.
- Place one pair of electrons between the central atom and each of the other atoms to form single bonds.
- Place the remaining electrons around the atoms in pairs to complete the octet rule. Hydrogen atoms only need two electrons to complete their valence shell.
- If the central atom does not have an octet, form double or triple bonds with the surrounding atoms to achieve an octet.
- Check that the total number of valence electrons in the Lewis structure is the same as the original count.
Lewis structures can be used to predict the shape of a molecule, its polarity, and its reactivity. They are an important tool for understanding the behavior of molecules in chemical reactions.
- 4.3 Molecular Geometry
Molecular geometry refers to the three-dimensional arrangement of atoms in a molecule. The shape of a molecule is determined by the arrangement of its atoms, which is in turn determined by the number of atoms and the nature of the chemical bonds between them.
The shape of a molecule is important because it affects the molecule's physical and chemical properties, such as its melting and boiling points, solubility, and reactivity.
There are several factors that determine the molecular geometry of a molecule:
- The number of electron groups around the central atom. An electron group is a lone pair of electrons or a bonding pair of electrons.
- The type of electron group. Bonding pairs of electrons repel each other more strongly than lone pairs, leading to different molecular shapes.
- The electronegativity of the atoms. Atoms with higher electronegativities will pull electrons more strongly towards themselves, leading to differences in bond angles and molecular shapes.
Some common molecular geometries include:
- ==Linear - two== atoms with a bond angle of 180 degrees, such as in ==carbon dioxide (CO2).==
- ==Trigonal planar -== three atoms with a bond angle of 120 degrees, such as in ==boron trifluoride (BF3).==
- ==Tetrahedral - four== atoms with a bond angle of 109.5 degrees, such as in methane ==(CH4).==
- Trigonal bipyramidal - five atoms with bond angles of 90 and 120 degrees, such as in phosphorus pentafluoride ==(PF5).==
- ==Octahedral - six== atoms with a bond angle of 90 degrees, such as in sulfur hexafluoride ==(SF6).==
These molecular geometries can also have variations, such as bent, trigonal pyramidal, or seesaw shapes, depending on the number and type of lone pairs present in the molecule.
- 4.4 Molecular Polarity
Molecular polarity refers to the uneven distribution of electron density within a molecule, which can result in a molecule having a positive and a negative end, similar to the way a magnet has a north and a south pole. The polarity of a molecule is important because it affects the molecule's interactions with other molecules, including its solubility and reactivity.
A molecule can be polar or nonpolar, depending on its molecular geometry and the polarity of its bonds. In a polar molecule, there is an uneven distribution of electrons, with one end of the molecule having a partial positive charge and the other end having a partial negative charge. In a nonpolar molecule, there is an even distribution of electrons, resulting in no overall polarity.
The polarity of a molecule can be determined by looking at the polarity of its bonds and its molecular geometry. If a molecule has polar bonds, it will be polar if the bond dipoles do not cancel each other out due to the molecular geometry. For example, water ==(H2O)== has polar bonds due to the electronegativity difference between hydrogen and oxygen, and the molecular geometry ==(a bent shape)== results in an uneven distribution of electrons, making water a polar molecule.
On the other hand, a molecule like carbon dioxide ==(CO2)== has polar bonds due to the electronegativity difference between carbon and oxygen, but the linear molecular geometry results in an even distribution of electrons, making ==CO2== a nonpolar molecule.
The polarity of a molecule can be measured experimentally using techniques such as infrared spectroscopy or by calculating its dipole moment.
- 4.5 Covalent Bond Formation
Covalent bond formation occurs when two or more atoms share electrons in order to achieve a more stable electron configuration. This type of bond typically forms between nonmetallic elements that have high electronegativities, which means they have a strong attraction for electrons.
The process of covalent bond formation involves the following steps:
- The atoms involved in the bond formation approach each other and their valence electrons interact.
- The valence electrons are shared between the atoms in order to fill their valence electron shells and achieve a more stable electron configuration.
- The shared electrons are attracted to the positively charged nuclei of both atoms, forming a bond.
- The strength of the bond is determined by the number of electrons shared and the distance between the nuclei of the atoms involved.
Covalent bonds can be classified as either single, double, or triple bonds depending on the number of electron pairs shared between the atoms. In a single bond, only one pair of electrons is shared between the atoms, while in a double bond, two pairs of electrons are shared, and in a triple bond, three pairs of electrons are shared.
Covalent bonds can also be polar or nonpolar depending on the electronegativity difference between the atoms involved. When atoms with different electronegativities are bonded together, the electrons are not shared equally and the bond is polar. When the electronegativity difference is zero or very small, the bond is nonpolar.
Examples of covalent compounds include ==water (H2O), methane (CH4), carbon dioxide (CO2), and nitrogen gas (N2).== Covalent compounds can have a wide range of physical and chemical properties, depending on the type and strength of the covalent bond, and the molecular structure of the compound.
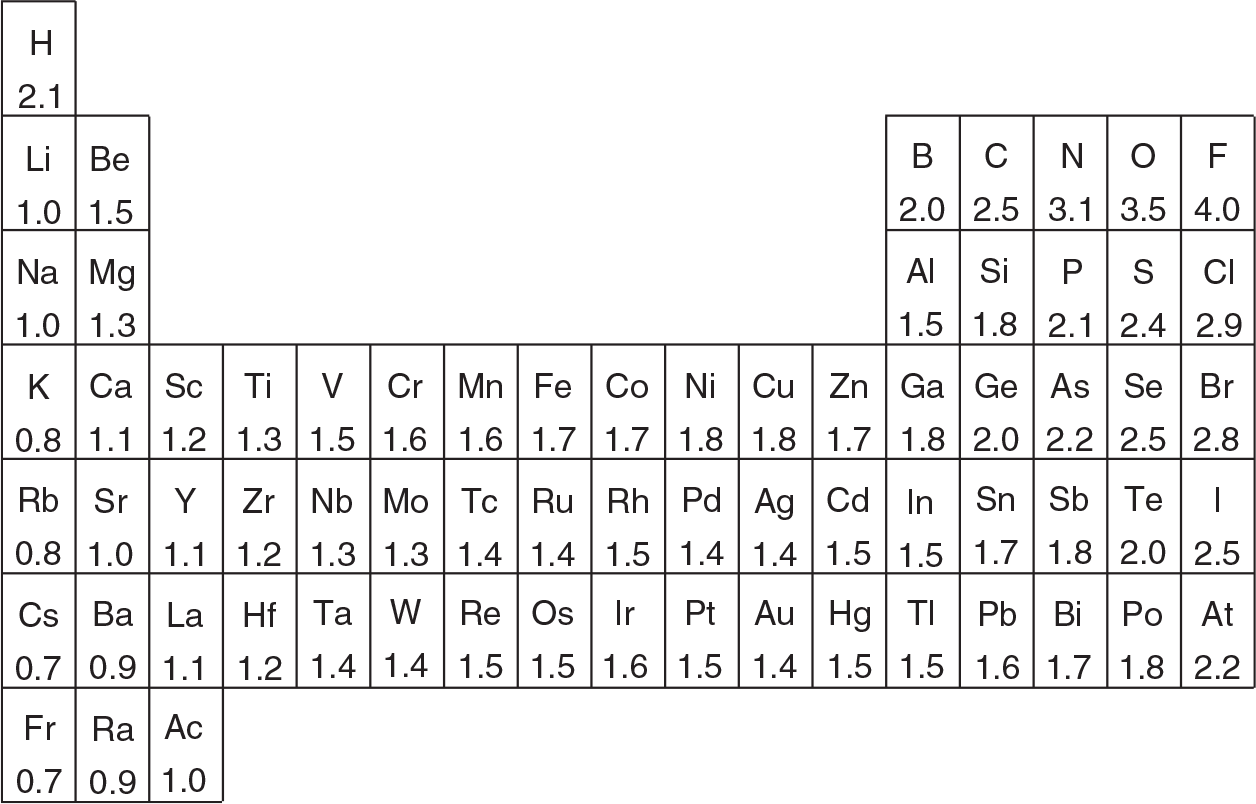
(a) Diethyl ether, CH3CH2OCH2CH3, is a highly flammable liquid. It is a common solvent in many laboratories.
Draw the Lewis structure for this compound. Identify the electron pairs and molecular geometry of the oxygen. Explain your response.
Based on the structure, the electron pair geometry around the oxygen atom in diethyl ether is tetrahedral and the molecular geometry is bent or angular. This is due to the two lone pairs of electrons on the oxygen atom.
Explain using VSEPR theory why the bond angle for the C–O–C bonds are not at 180°.
This molecule has an ==AX2E2 shape, where there are 2 atoms and 2 nonbonding electron== pairs on the central oxygen atom. According to VSEPR theory, these 4 electrons repel each other in such a way as to be as far apart as possible. Nonbonding electron pairs require more space than do bonding electrons. Therefore the bond angle in this molecule is much smaller than the usual angle of ==109.5°== for a tetrahedron arrangement.
Are there any polar bonds in this molecule? If so, indicate the positive and negative poles of each bond. Is this molecule polar or nonpolar? Defend your answer.
The two ==C–O bonds== are polar due to the difference in electronegativity between the two atoms. The molecule is also polar since it is asymmetrical and the polarities of the individual ==C–O bonds== do not cancel out each other.
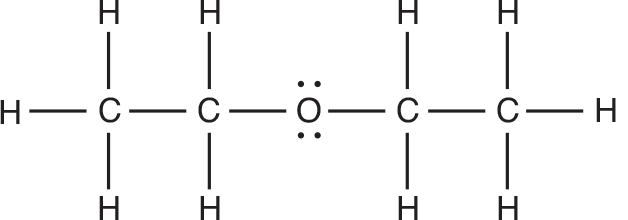
- Draw the Lewis structure for nitrogen trichloride, and determine its molecular geometry. Explain what hybrid orbitals are required to produce this structure.
The molecular geometry for this molecule is trigonal pyramid due to the nonbonding pair of electrons on the nitrogen. However, the basic structure pattern for ==NCl3 is AX3E,== which is tetrahedron. In order to form the tetrahedron electron pair geometry, 4 sp3 hybridized orbitals are necessary.
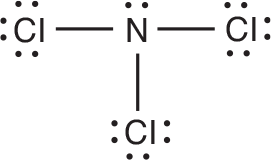
2. Based on the structure of nitrogen trichloride, are the bonds in this molecule polar? If so, indicate the positive and negative poles in an appropriate manner. Explain your reasoning.
The ==N–Cl== bonds in this molecule are polar because of the significant difference in electronegativity between ==Cl and N.== We should indicate the polarity of the bond as shown in the following diagram.The partial ==sign, δ,== is used to symbolize a partial charge because neither a complete positive charge nor a complete negative charge is formed. 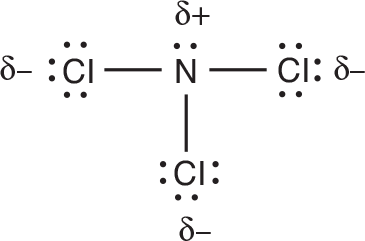
3. As seen in part (i), a molecule of nitrogen trichloride is possible. Is a molecule of nitrogen pentachloride possible? Justify your answer.
A molecule of nitrogen pentachloride is not possible since the nitrogen atom is too small to fit all ==5 chlorines around it.== This molecule would require an expanded octet, and the nitrogen atom does not have the ability to do so.
Sure, here are the Lewis structures for each of the compounds you mentioned:
- CH3Cl:
cssCopy code
H Cl | | H-C---C | H
The carbon is the central atom with single bonds to three hydrogen atoms and one chlorine atom. There is also a lone pair of electrons on the chlorine atom.
- CS2:
Copy code
S=C=S
The sulfur atom is the central atom with double bonds to two carbon atoms.
- PH3:
cssCopy code
H | H-P-H | H
The phosphorus atom is the central atom with single bonds to three hydrogen atoms. There is also a lone pair of electrons on the phosphorus atom.
- SiF4:
rCopy code
F F | | F-Si---Si-F | | F F
The silicon atom is the central atom with single bonds to four fluorine atoms.
- H2S:
cssCopy code
H | H--S--H | H
The sulfur atom is the central atom with single bonds to two hydrogen atoms. There is also a lone pair of electrons on the sulfur atom.
In terms of electron pairs and molecular geometry, here is a summary for each compound:
- CH3Cl: tetrahedral electron pair geometry, tetrahedral molecular geometry
- CS2: linear electron pair geometry, linear molecular geometry
- PH3: tetrahedral electron pair geometry, pyramidal molecular geometry
- SiF4: tetrahedral electron pair geometry, tetrahedral molecular geometry
- H2S: tetrahedral electron pair geometry, bent (V-shaped) molecular geometry.
Construct the Lewis structure for each of the following ions.
Sure, here are the Lewis structures for each of the ions you mentioned:
- NH4+:
yamlCopy code
H H | | H-N-H H+ | | H H
The nitrogen atom is the central atom with single bonds to four hydrogen atoms. The positive charge is located on the nitrogen atom.
- SO3^2-:
cssCopy code
O || O--S--O-- || O
The sulfur atom is the central atom with double bonds to two oxygen atoms and a single bond to another oxygen atom. The overall charge of the ion is 2-.
- CO3^2-:
cssCopy code
O || O--C--O-- || O
The carbon atom is the central atom with double bonds to two oxygen atoms and a single bond to another oxygen atom. The overall charge of the ion is 2-.
- CN-:
cssCopy code
C || N--C || C
The nitrogen atom is the central atom with a triple bond to the carbon atom. The overall charge of the ion is 1-.
In each of these Lewis structures, the valence electrons are represented as dots or lines between atoms, and each atom has a full valence shell (except for hydrogen, which has two electrons in its valence shell) by sharing electrons with neighboring atoms.
From this structure we calculate the formal charges on sulfur and oxygen:
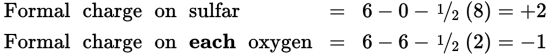
We find that the formal charge on each oxygen is −1 and the formal charge on sulfur is +2. These charges add up to the charge of the ion, as they should (+2 −1 −1 −1 −1 = −2). These formal charges are large, and an alternative structure should be sought. The structure shown in Figure 4.14 is one possible variation
There are now two types of oxygen-sulfur bonds (two with a single bond and two with a double bond). When the formal charges are calculated, we have these results:
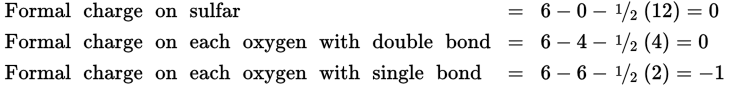
This is the preferred structure since it has the minimum formal charges. The formal charges add up to the charge of the ion (−1 −1 = −2) and cannot be any lower.
Formal charges may also be used to deduce the appropriate structure for a compound that has many possible Lewis structures. For instance, the NOCl molecule can be drawn with the four structures shown in Figure 4.15.
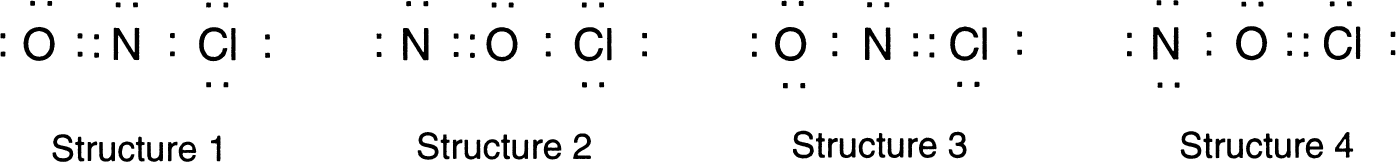
To determine which of these structures is the most reasonable, we calculate the formal charges. Using the rules for determining these charges, we obtain the values in Table 4.2.
| Element | Structure 1 | Structure 2 | Structure 3 | Structure 4 |
|---|---|---|---|---|
| Oxygen | 0 | +1 | –1 | +1 |
| Nitrogen | 0 | –1 | 0 | –2 |
| Chlorine | 0 | 0 | +1 |
Table 4.2 Formal Charges on NOCl Structures
From Table 4.2 we see that the first structure has the lowest formal charges on all atoms, and it is preferred. Structure 2 has larger formal charges but also has a negative charge on nitrogen even though nitrogen is less electronegative than oxygen. This is not a reasonable situation since the more electronegative atom is expected to have the more negative formal charge. Structures 3 and 4 also have negative charges on the less electronegative elements as well as formal charges greater than zero. We therefore conclude that structure 1 is the most reasonable structure.
Exercise 4.2
In Exercise 4.1 the Lewis structures of the molecules and ions listed below were constructed. Now calculate the formal charges for each of those structures. Also, suggest which ones may have better structures, and draw them.
- CH3Cl
- CS2
- PH3
- SiF4
- H2S
Solution
all zero
all zero
all zero
all zero
all zero
Exercise 4.3
In Exercises 4.1 and 4.2 simple Lewis structures were constructed and then modified, based on the formal charges. Use those structures to draw the resonance structures of the following ions:
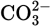
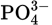
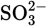
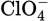
Solution
You should find three resonance structures each for (a), (b), and (d) and four resonance structures each for (c) and (e).
Exercise 4.4
Without using Figure 4.20, indicate the positive and negative ends of each of the following bonds by the symbols δ+ and δ−:
- S–O
- C–N
- S–P
- C–F
- Si–O
- H–Br
- H–O
Solution
Using the diagonal relationships in the periodic table, we identify the positive and negative ends as follows:
- δ+S–Oδ−
- δ+C–Nδ−
- δ−S–Pδ+
- δ+C–Fδ−
- δ+Si–Oδ−
- δ+H–Brδ−
- δ+H–Oδ−
The electronegativity table in Figure 4.20 gives numerical data that may be used to evaluate the magnitude of bond polarity. This is done by taking the absolute value of the difference in the electronegativities of the two atoms participating in a bond. We may call this value delta EN or, in written form, ∆EN:

The larger the value of ∆EN, the greater the polarity of the bond. If ∆EN is zero, the bond is considered to be nonpolar.
Exercise 4.5
For the bonds in Exercise 4.4, determine the ∆EN values, and predict which bond is the least polar and which is the most polar.
Solution
- 1.1
- 0.6
- 0.3
- 1.5
- 1.7
- 0.7
- 1.4
The Si–O bond is the most polar, and the S–P bond is the least polar.
Without an electronegativity table, it is possible to determine which of two bonds is the more polar if the bonds have one atom in common. The more polar bond will be the one where the second atom is furthest in the periodic table from the common atom. For instance, the nitrogen-fluorine bond is more polar than the oxygen-fluorine bond since nitrogen is further from fluorine than is oxygen.
Exercise 4.6
Using a periodic table, but not a table of electronegativities, estimate, for each of the following pairs, which bond is more polar:
- C–N or C–O
- H–Cl or H–Br
- S–O or S–Br
- H–S or H–O
- P–Br or S–Br
Solution
The more polar bond in each pair belongs to (a) C–O, (b) H–Cl, (c) S–O, (d) H–O, and (e) P–Br.
Exercise 4.7
Determine the bond order of the central atom of each of the following compounds. You may use the structures determined in Exercises 4.1 and 4.2.
- CH3Cl
- CS2
- PH3
- SiF4
- H2S
Solution
If we use the simple Lewis structures, we obtain
- 1
- 2
- 1
- 1
- 1
If we use the best formal charge structures, we obtain
1
2
1
1
1
Exercise 4.8
Name each of the following covalent molecules:
- SO2
- P4O10
- N2O3
- SF4
- PBr5
- XeF4
- BrCl3
- S4N4
Solution
sulfur dioxide
tetra phosphorus dioxide
dinitrogen trioxide
sulfur tetrafluoride
phosphorus pentabromide
xenon tetrafluoride
bromine trichloride
tetra sulfur tetranitride
Exercise 4.9
Give the formula for each of the following compounds:
- diaoron tetrabromide
- boron trifluoride
- carbon tetrafluoride
- carbon monoxide
- diphosphorus pentoxide
- carbon disulfide
- sulfur trioxide
- nitrogen triiodide
Solution
- B2Br4
- BF3
- CF4
- CO
- P2O5
- CS2
- SO3
- NI3
Additional rules and names for compounds are given in Chapter 13 on acids and bases.