structure of matter
Dalton’s atomic theory states that:
All matter is composed of tiny, indivisible particles, called atoms, that cannot be destroyed or created.
Each element has atoms that are identical to each other in all of their properties, and these properties are different from the properties of all other atoms.
Chemical reactions are simple rearrangements of atoms from one combination to another in small whole-number ratios.
Atomic Models
Solid Particle model
Plum pudding model: electrons bathed in a sea of positive charges like raisins in pudding
Nuclear model Rutherford: positively charged center with a lot of space and some electrons ( came after the gold foil experiment)
Solar system Bohr: electrons exist in fixed orbitals
Wave-mechanical their Schrödinger: similar to Bohr but the position of the electron in the wave-mechanical model is described by a probability of where it will be located (electron cloud)
Atomic Structure
An atom (or molecule) usually exists in the lowest possible energy state, which is called the ground state.
An atom (or molecule) that has more energy than the ground state is said to be in an excited state.
When an atom loses energy in going from an excited state to a ground state, light may be emitted.
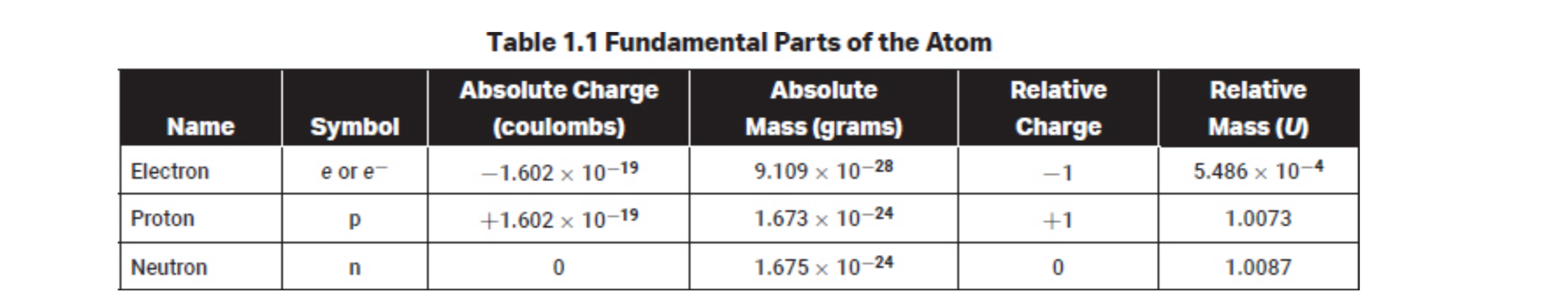
The energy, wavelength, frequency and speed of light are related through planks equation
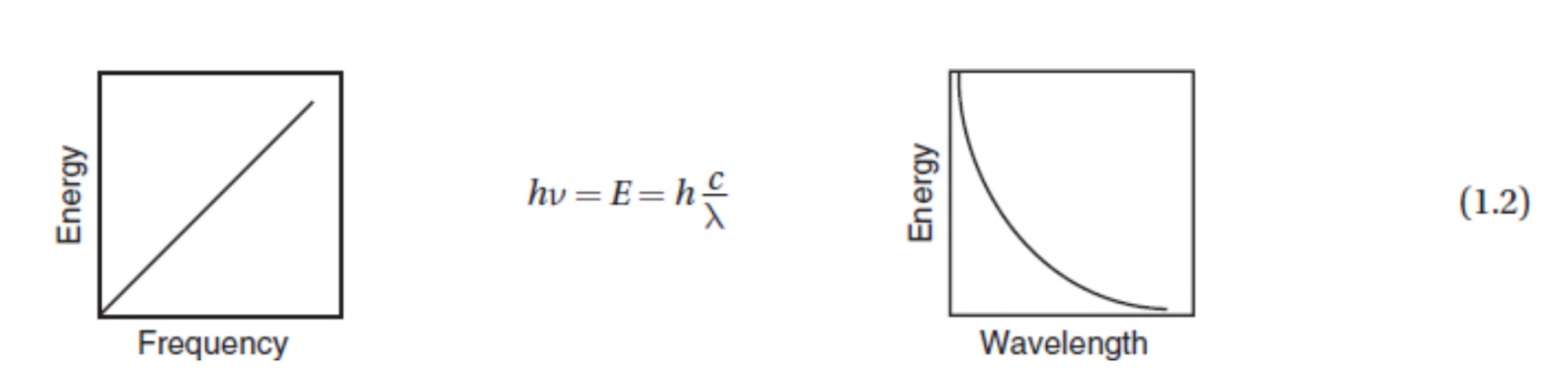
Where E is energy, c is the speed of light in a vacuum, v is the frequency, and h is planks constant with a value of 6.63 × 10^-34 joule second
The most stable position for the electron in the atom is the first level since the electron has the lowest possible amount of energy
Bohr’s model
Bohr’s model requires that electrons stay in fixed orbitals
Light is emitted when an electron travels to a lower level and energy is absorbed when electron travels to higher level
Energy released from a high level to a low level is the same as the energy absorbed to go from a low level to a high level
53 is Bohr radius for H and other elements are whole number multiples of it (gives estimate for size)
Wave-Particle Duality
Electrons can act as both waves and particles which allows for this equation
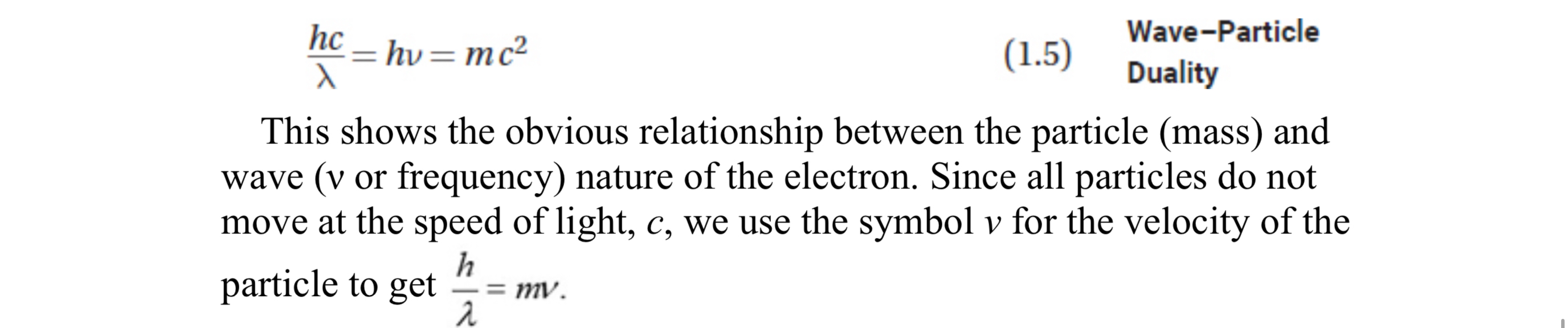
Wave equations are needed to describe an electrons motion as a wave
Describing electrons as waves leads to the following:
Quantum numbers n,l,ml, & ms are needed to solve wave equations
Electrons as waves causes Bohr’s orbitals to be replaced by an electron cloud where the modern orbit is the region of space in which the probability of finding the electron is highest.
The wave equations have shown that the shapes of most electron clouds, although more complex than Bohr’s orbits, are still simple geometric shapes. (circular orbits turn into spherical orbits)
The arrangement of electrons deduced from wave equations agrees well with the periodic table. Many physical and chemical properties of elements and compounds are more fully understood with the knowledge gained about the electronic structure and orbit shape.
The results of the wave equations agree completely with the Bohr model. Specifically, the energy change for an electron moving from one electron cloud to another agrees with Bohr’s calculations. In addition, the identical 53 pm radius is found for the electron cloud in the wave model of the hydrogen atom.
The Heisenberg uncertainty principle is fundamental to the wave model of the atom. This principle states that both the position and the momentum of an electron cannot be exactly known at the same time. The more precisely that the position, x, of the electron is known, the more uncertainty exists as to its momentum, mv.
Structure of an Atom
Quantum Numbers
Quantum numbers basically describe the location of an electron in the electron cloud