IB Chemistry Atoms and electrons 2025
1/65
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
66 Terms
Define first Ionization energy
the energy required to remove one electron from each atom in one mole of gaseous atoms under standard conditions
Define Second Ionization energy
the energy required to remove one electron from one mole of gaseous 1+ ions under standard conditions
equation for first ionization energy of magnesium
M(g) → M⁺(g)+e⁻
equation for second ionization of magnesium
M ⁺(g) → M²⁺(g)+e⁻
Why is second ionization energy always higher than the first?
positive ions attract electrons more strongly than neutral atoms there is less repulsion between remaining electron after an electron has been removed (stronger attraction to the nucleus)
What is shielding?
decrease in attraction between a (valence) electron and the nucleus due to repulsion from inner electrons
Effect of more shielding
decrease in ionization energy as there is less attraction between the valence electrons and the nucleus
Effective nuclear charge
the net attractive positive charge of nuclear protons acting on valence electrons
Effect of higher effective nuclear charge
increase in ionization energy as there is greater attraction between the nucleus and the valence electrons
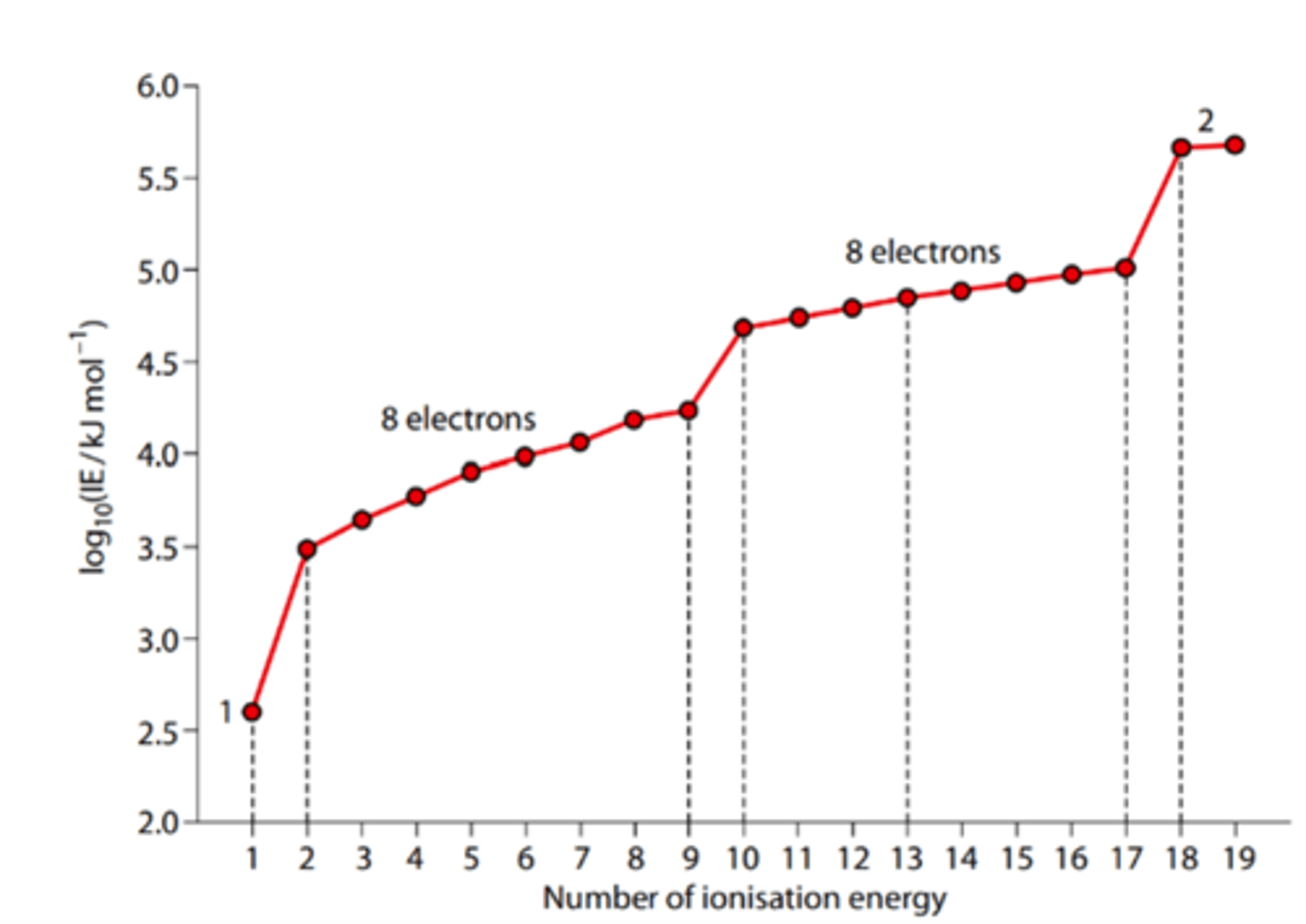
Sketch the graph of the successive ionization energies (IE) of Potassium
What is the equation for photon energy (E)
E=hv from h (Planck's constant) and f (frequency of the light in the emission spectrum)
How does ionization energy vary across a period
it generally increases as there is a larger effective nuclear charge felt by electrons in the same shell.
How does ionization energy vary across a group
it decreases as atomic radius increases and thus attraction between the nucleus and the valence electrons decreases.
equation for wavelength (λ)
λ=c /f from the speed of light (c) divided by frequency (f)
Explain why the third ionization energy of magnesium (Mg) is much greater than the second ionization energy
The electron is in lower energy level, so the electron shell is more stable OR The electron closer to nucleus, so there is an increase in effective nuclear charge. AND less shielding as fewer shells
Why is there a drop in ionisation energy between group 15 and 16?
Electron pair repulsion due to electrons sharing a p orbital
Why is there a drop in ionisation energy from Be to B?
The highest energy electron in B is in a p orbital which is at a higher energy level than the s orbital
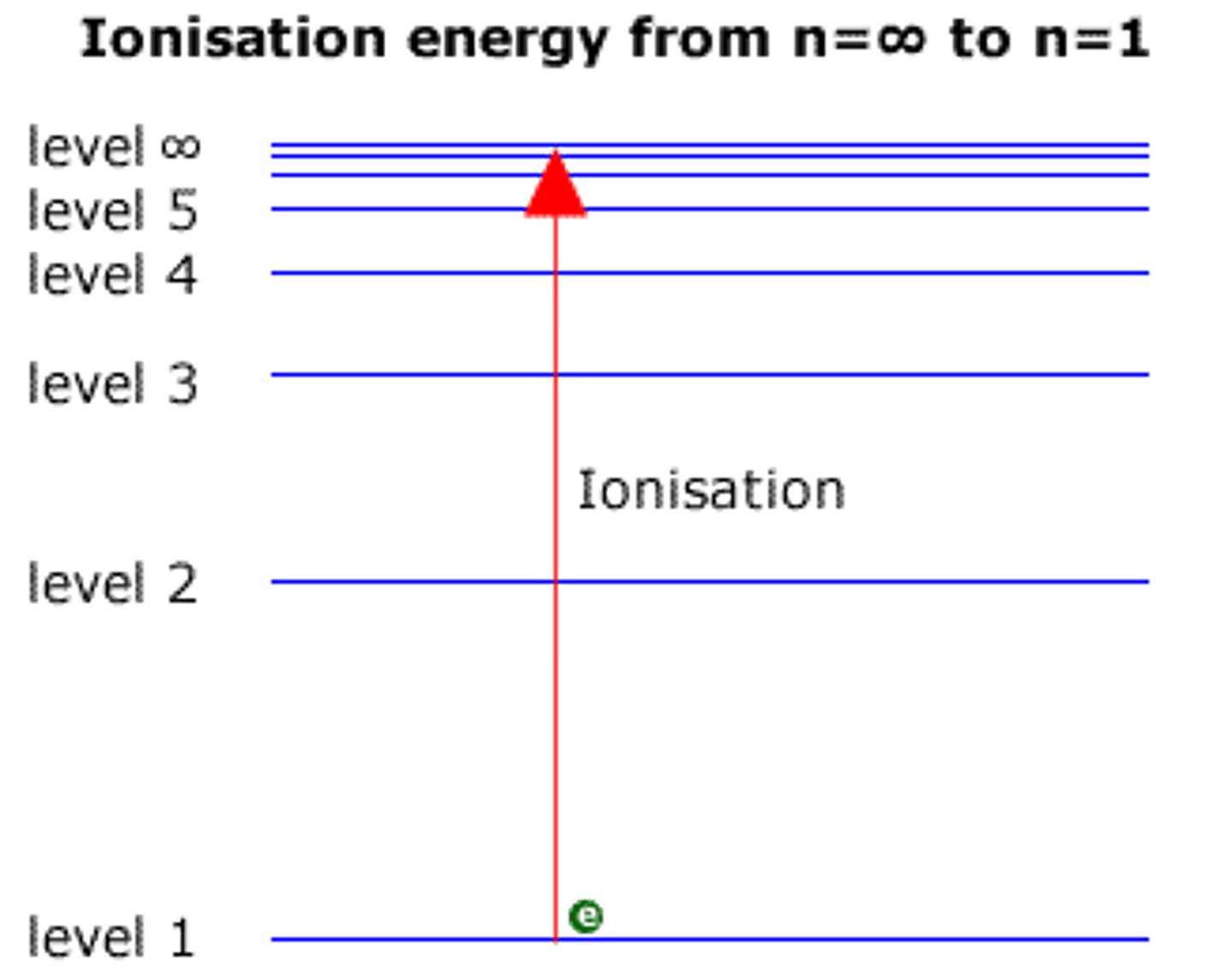
The ionisation energy is the energy transition between an electron and ....
the convergence limit (n=∞)
Electronegativity
a measure of the tendency of an atom to attract a bonding pair of electrons
electron affinity
the energy change that occurs when an electron is acquired by a neutral atom
Periodicity
the repeating pattern of chemical and physical properties of the elements
line spectrum
a spectrum showing only certain discrete wavelengths
continuous spectrum
an emission spectrum that consists of a continuum of wavelengths.
Isotope
Atoms of the same element that have different numbers of neutrons
Emission spectra are produced by
atoms emitting photons when electrons in excited states return to lower energy levels.
A transition in the hydrogen atom to n = 3 produces
Infrared light
A transition in the hydrogen atom to n = 2 produces
visible light
A transition in the hydrogen atom to n = 1 produces
Ultra violet light
The main energy level is given an integer number, n, and can hold a maximum of
2n² electrons
Shape of an s orbital
spherical
shape of p orbital
dumbbell
group 1
Alkali metals
Group 17
Halogens
Group 0
noble gases
Define oxidation
oxygen gain/hydrogen loss, electron loss or increase in oxidation number.
Which elements have variable oxidation state
transition metals and most main-group non-metals.
Oxidation state of elements
0
Oxidation state of fluorine
-1 in its compounds
oxidation state of oxygen
-2 except in peroxides where it is -1
oxidation state of chlorine
-1 (except in compounds with F and O, where it has positive values)
oxidation state of hydrogen
Always +1 except in metal hydrides where it's -1
oxidation state of group 1
+1
oxidation state of group 2
+2
oxidation state of group 13
+3
The sum of the oxidation states in a species =
the charge of the species (0 in molecules)
Half equations show
either oxidation or reduction
In a reaction a reducing agent will be
oxidised
In a reaction an oxidising agent will be
reduced
which is correct for showing oxidation number 2+ or +2
+2
What does the (VI) in potassium dichromate (VI) tell us about the compound
The oxidation number (+6) of chromium
Oxidising agent
The reactant that is reduced (gains electrons)
reducing agent
The reactant that is oxidized (loses electrons)
electronic configuration of copper
1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰
electronic configuration of chromium
1s²2s²2p⁶3s²3p⁶4s¹3d⁵
Aufbau principle
electrons fill subshells of the lowest available energy, then they fill subshells of higher energy.
Hund's Rule
when an elecron is added to a subshell, it will always occupy an empty orbital if one is available
Pauli Exclusion Principle
The rule that an orbital can hold only two electrons with opposite spin
Relative atomic mass
The weighted average mass of an atom relative to relative to 1/12 of a carbon-12 atom
Relative formula mass
The weighted average mass of the formula of a compound relative to relative to 1/12 of a carbon-12 atom
Relative isotopic mass
The mass of an atom of an isotope on a scale where the mass of an atom relative to 1/12 of a carbon-12 atom (NOT AVERAGE)
In an emission spectrum, the limit of convergence at higher frequency corresponds to
ionization energy
What does hot gas give?
Hot gas causes emission spectrum because it excites electrons. (electron emits photon when deexcited)
What does cold gas give?
Cold gas gives absorption spectrum (electron absorbs EM wave so it is not detected)
Why do group 7 elements melting points increase down the group?
Molecules held together by Van der waals forces
Van der waals forces increases with increasing size of atom and more number of electrons
Steps for making aqueous solution (not sure if correct)
Put known mass of solid in volumetric flasK
Make up to line with water
Invert
Steps for diluting initial solution (not sure if correct)
use funnel to transfer initial solution without loss
Use dilution formula C1V1 = C2V2
Use volumentric flask
Transfer using volumetric pipette
[CHECK SCHOOLOGY FOR ADDITIONAL?]