6. Ion flux and the resting membrane potential
1/18
Earn XP
Description and Tags
Lecture 6
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
19 Terms
What creates the membrane potential in a neuron?
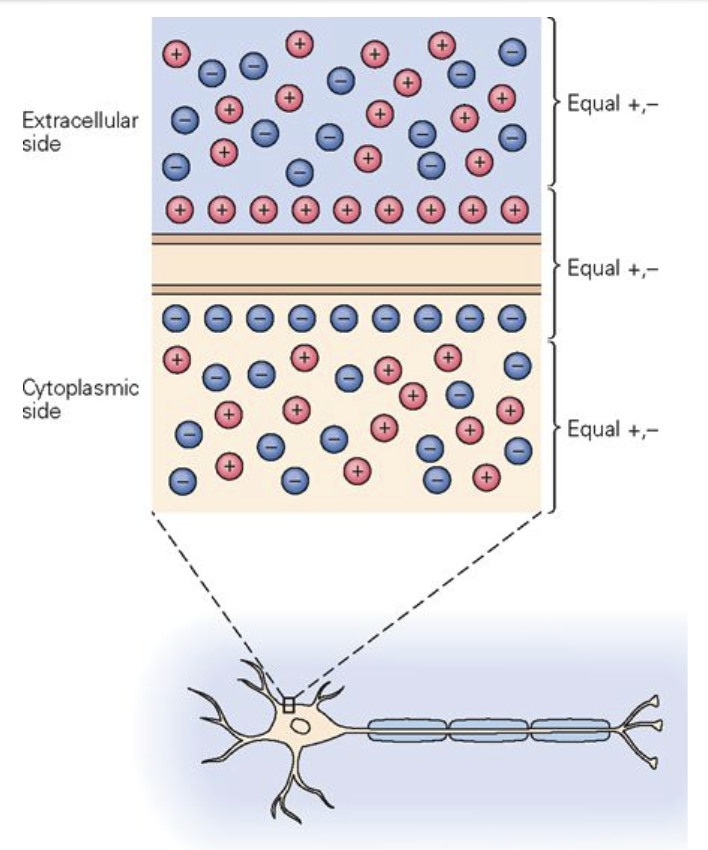
Separation of charges across the membrane: outside surface has excess positive charge, inside surface has excess negative charge → electrical potential difference.
However, the charges in the cytoplasm, and extracellular space are neutral.
What is membrane potential (Vm) and resting membrane potential?
Vm = Vin – Vout. At rest (~ –60 to –70 mV), there’s no net charge movement. Vm changes when ions flow across the membrane.
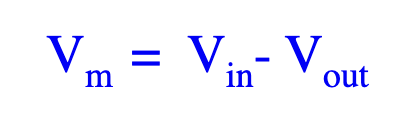
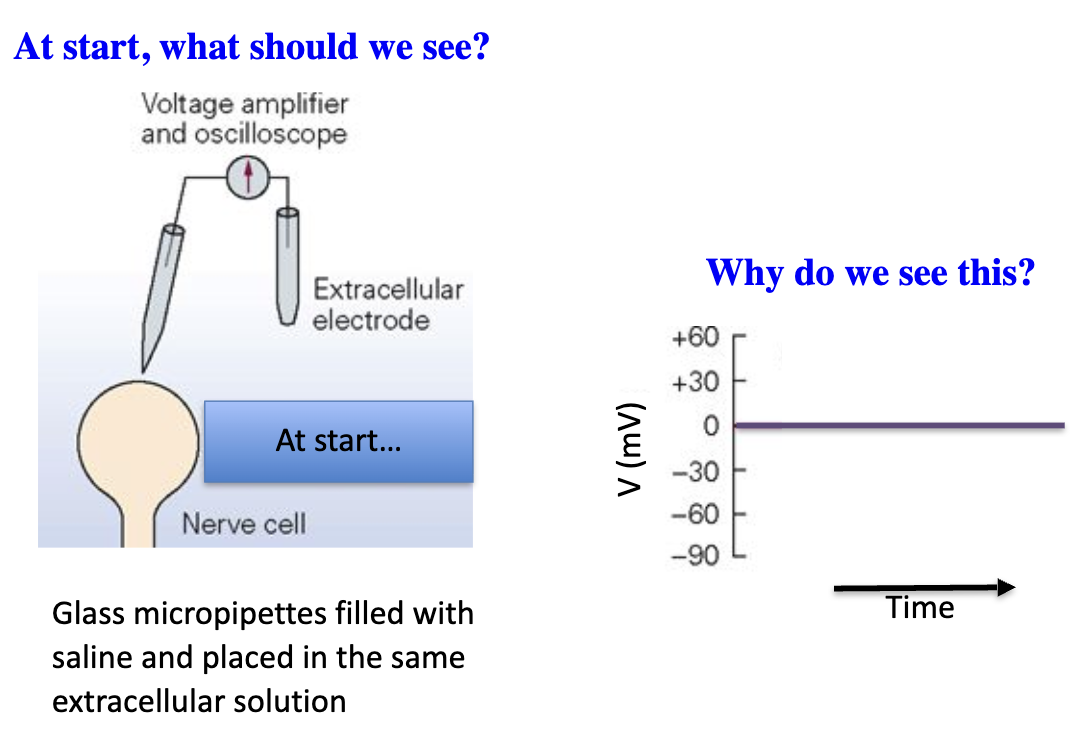
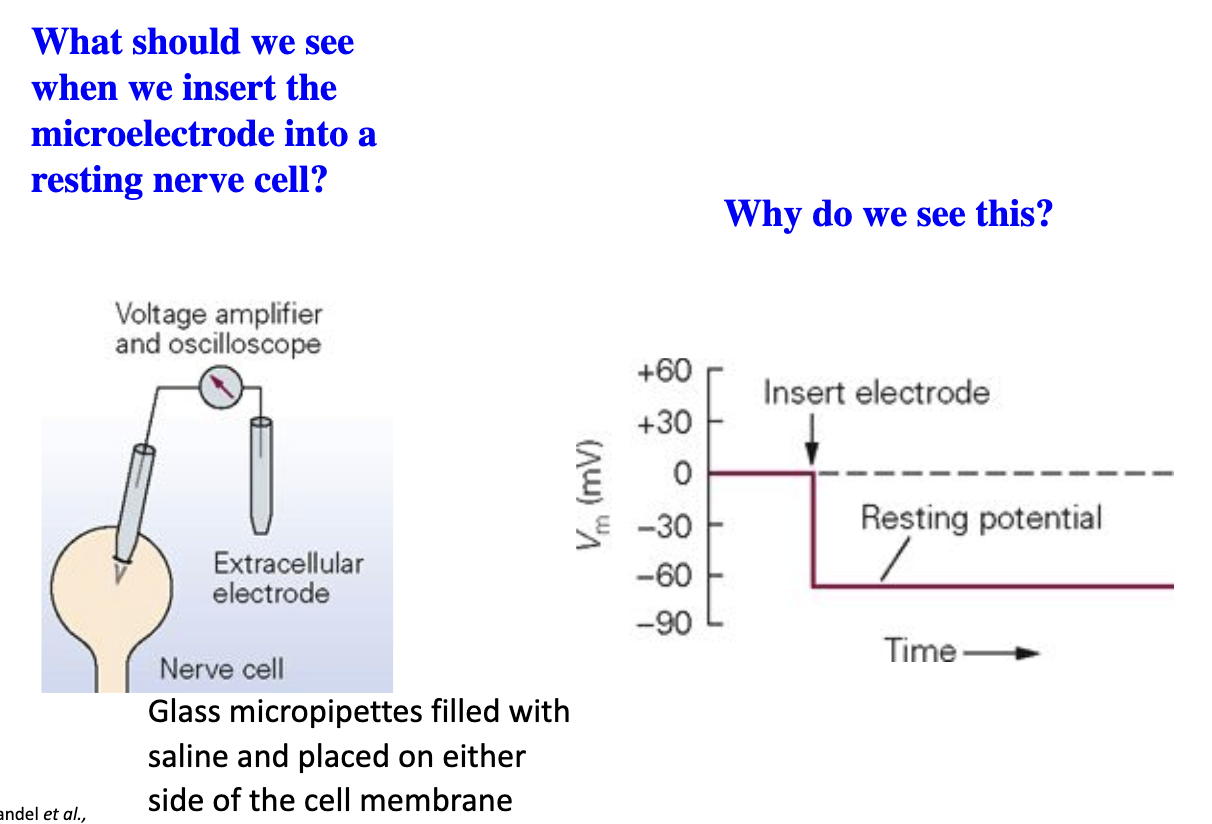
What happens when a microelectrode is placed in extracellular fluid?
Outside cell = 0 mV (same solution inside micropipette).
No separation in charge between the measuring electrode and reference electrode.
What happens when a microelectrode is placed inside a resting neuron?
Vm shifts to about –60 mV because the inside of the cell is negatively charged relative to the outside (resting membrane potential).
Charge difference between the reference electrode and recording electrode.
Isopotential: The voltage is the same across the cell.
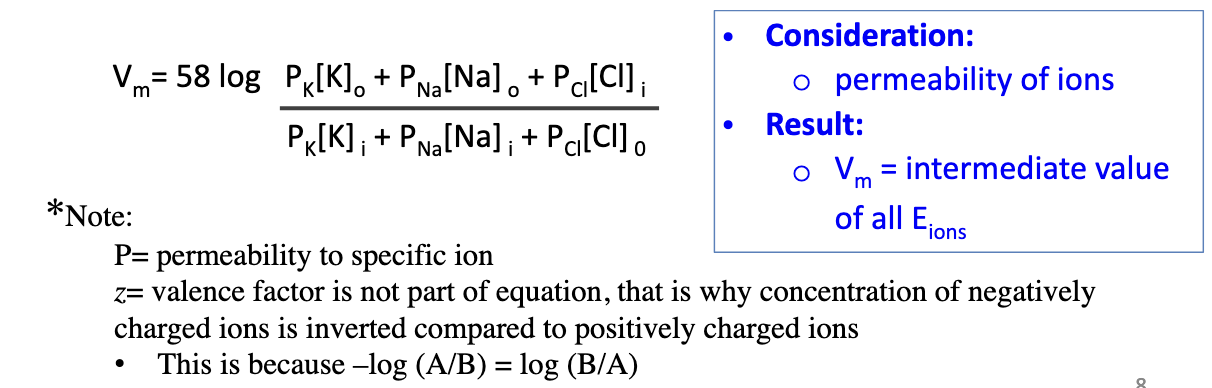
Why is the Goldman-Hodgkin-Katz (GHK) equation more accurate than Nernst?
Membranes are permeable to multiple ions (Na+, K+, Cl–), not just one. Vm reflects the combined effect of ion permeabilities.
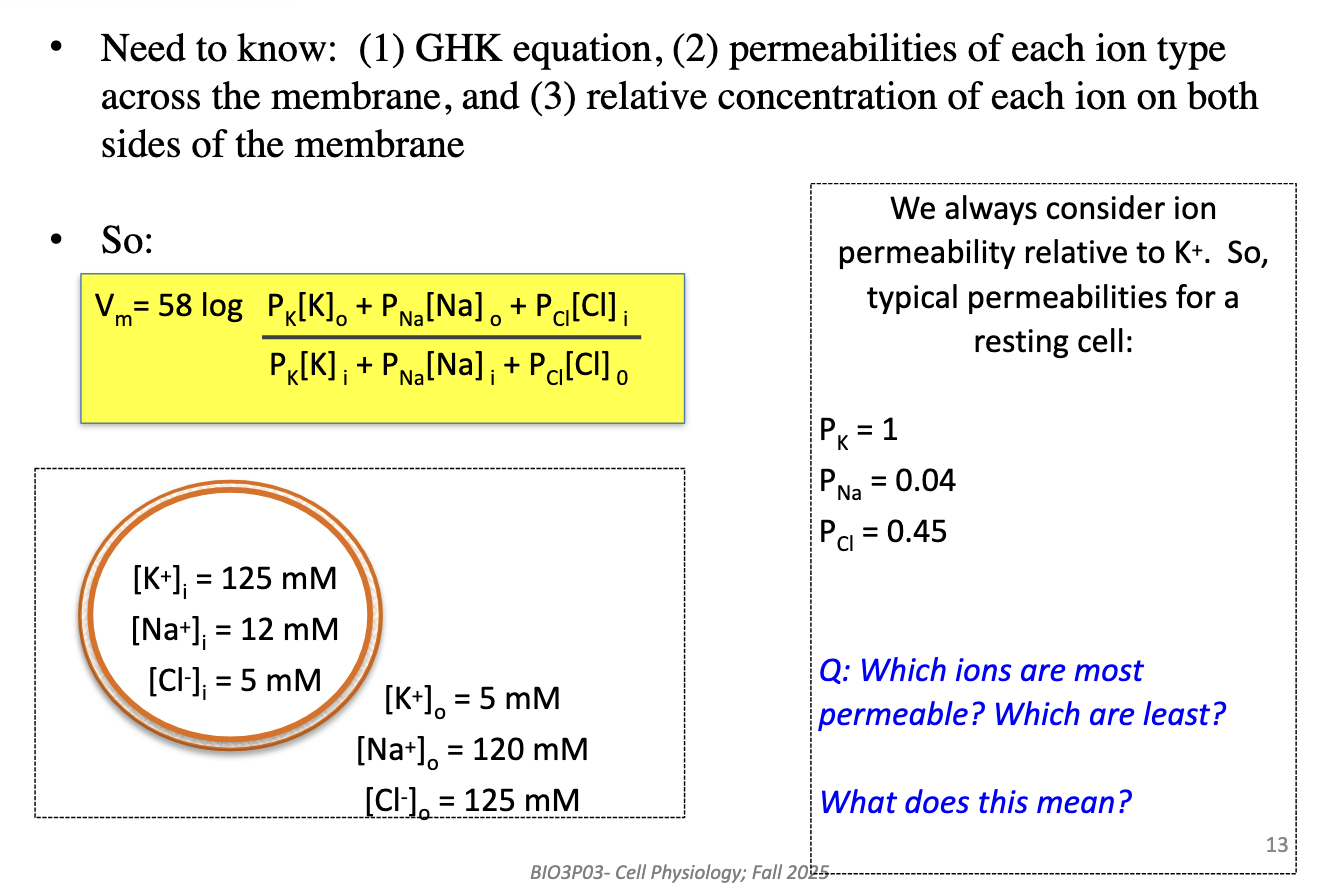
Which ions are most and least permeable in a resting neuron?
Most: K+ (1.0). Intermediate: Cl– (0.45). Least: Na+ (0.04). Vm is closest to K+ equilibrium because it dominates permeability.
All permeabilities are relative to K+
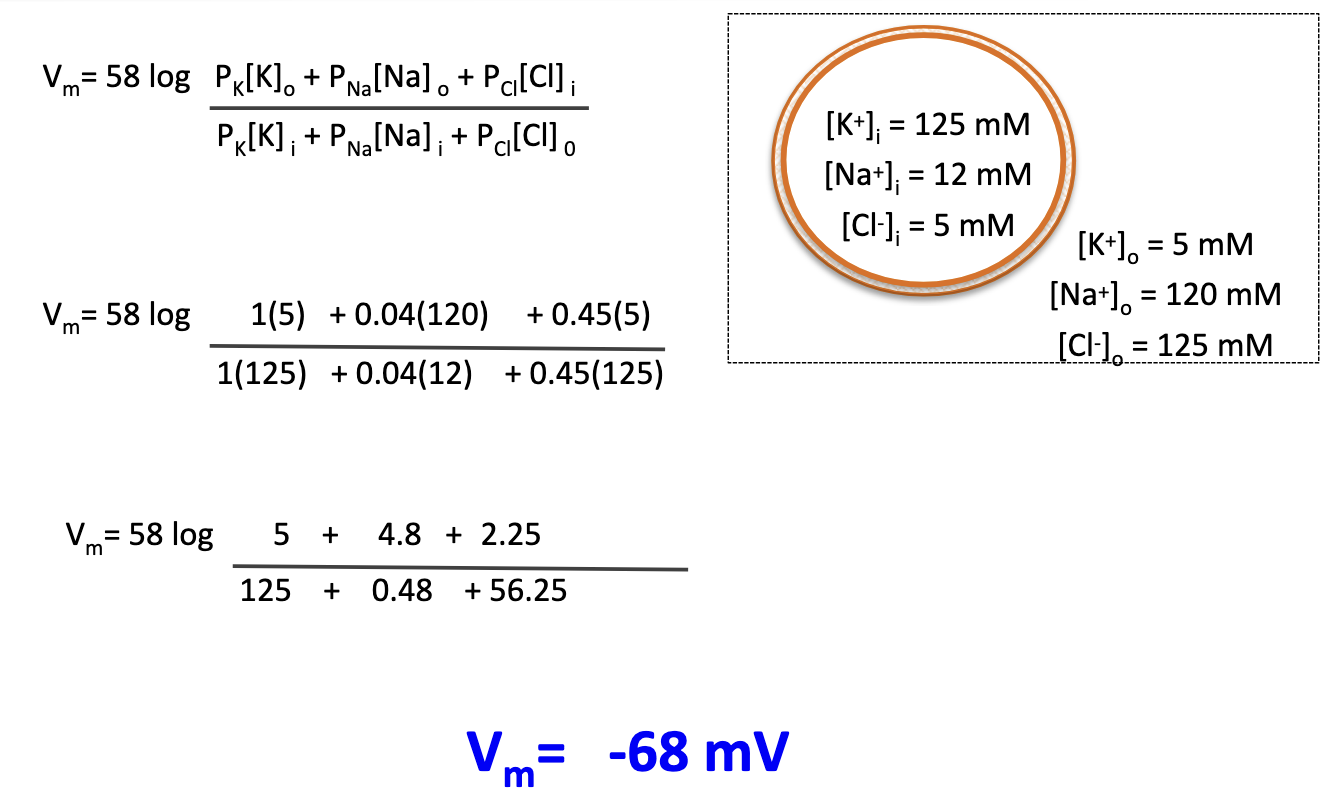
What is the typical Vm of a neuron at rest using GHK equation?
About –68 mV.
What does it mean if Vm ≈ EK and ECl but far from ENa?
Resting Vm is closer to K+ and Cl– equilibrium potentials. DRIVING force of an ion will try to force Vm towards Eion.
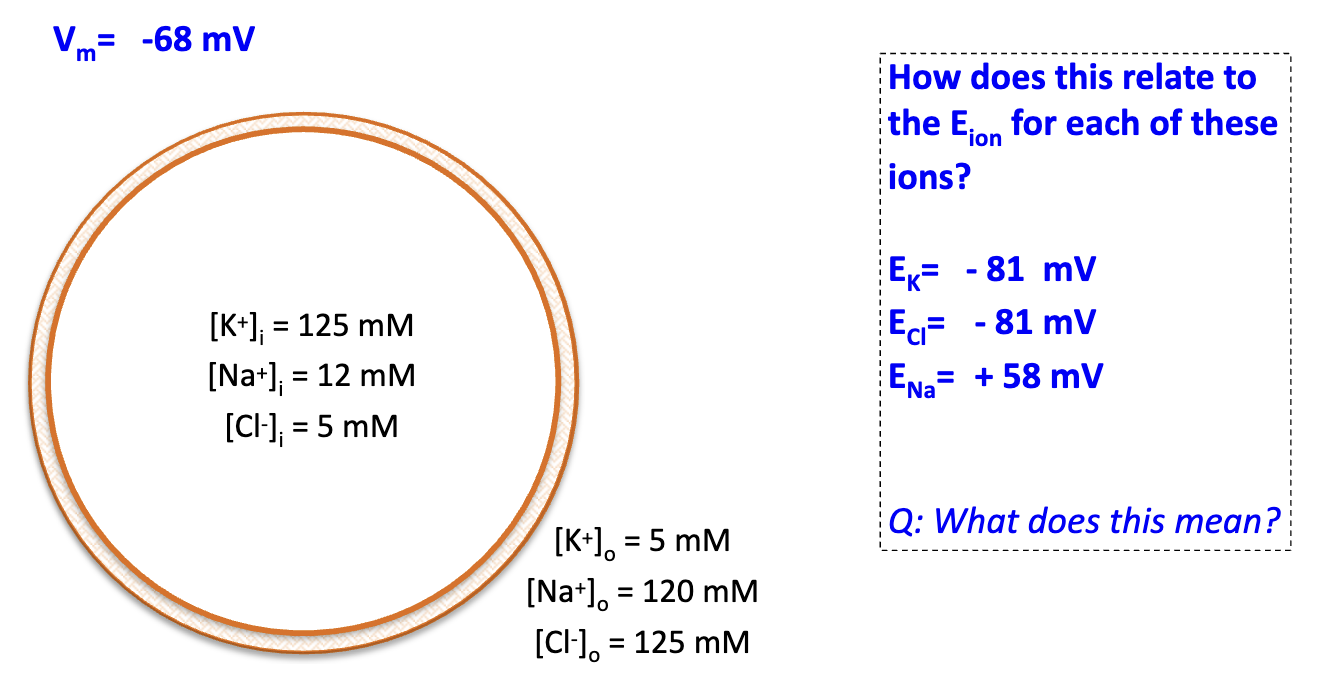
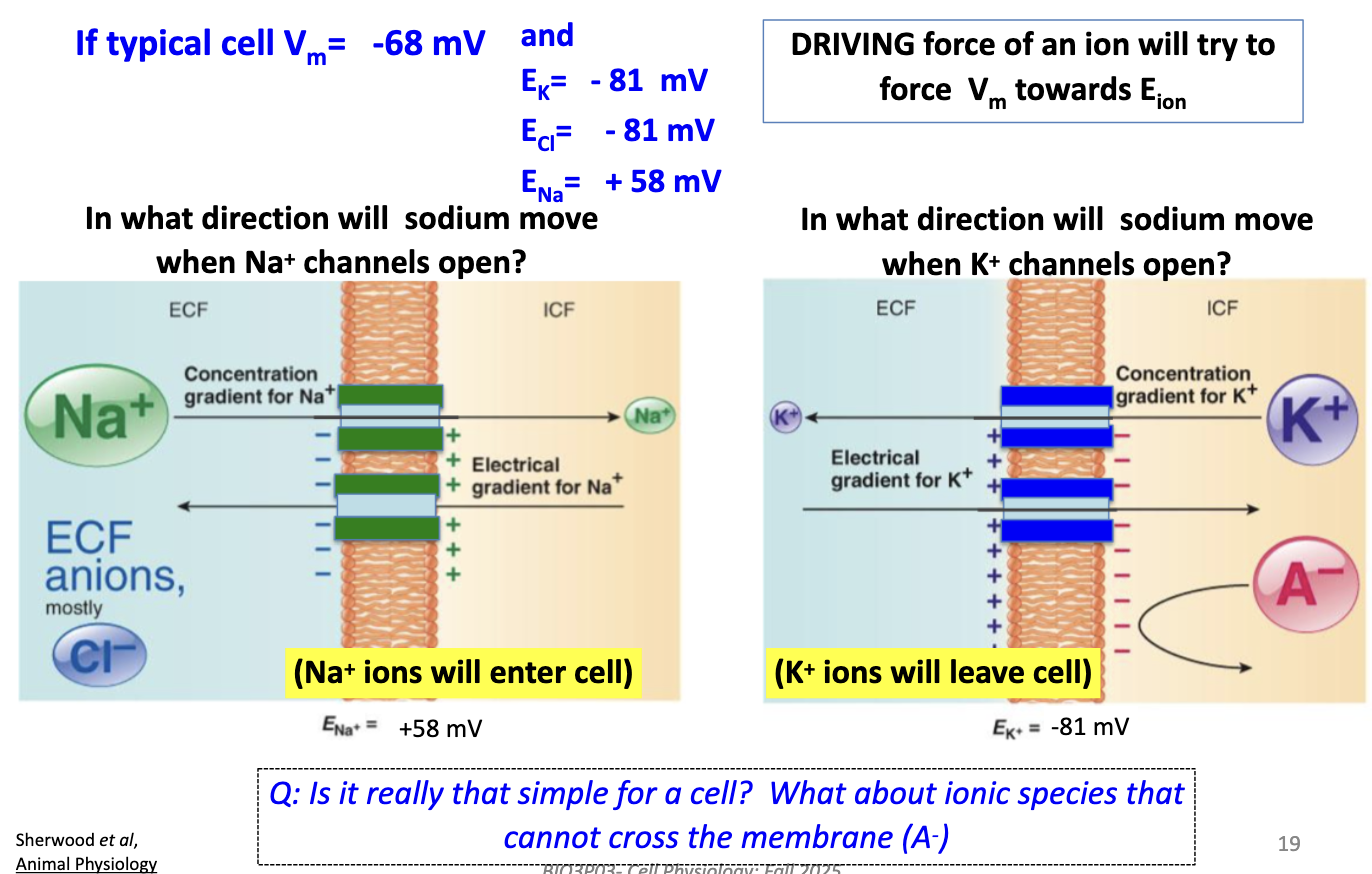
At Vm = –68 mV, how do ions move when channels open?
K+ leaves (drives Vm toward –81 mV). Na+ enters (drives Vm toward +58 mV). Cl– near equilibrium, little net movement.
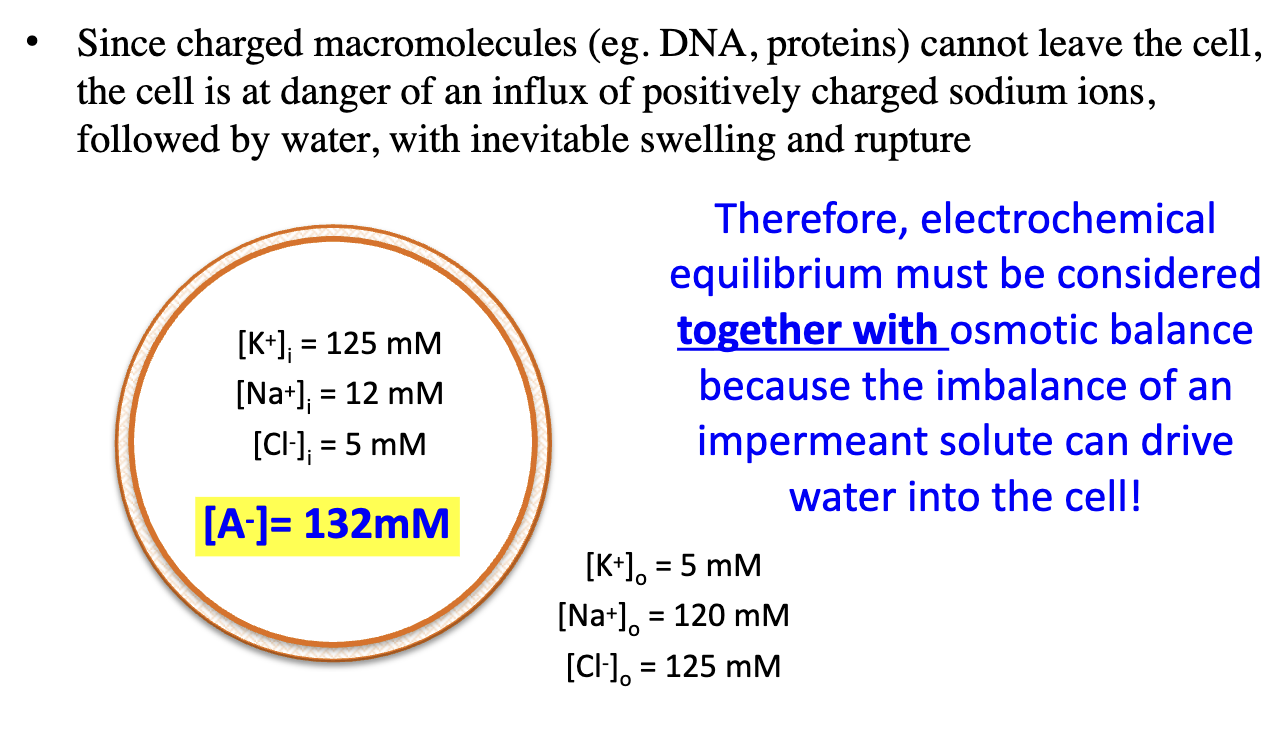
Why can impermeant anions (A–) be dangerous for cells?
They can trap cations inside, pulling in water → swelling and rupture unless balance is maintained.
Electrochemical equilibrium must be considered together with osmotic balance because the imbalance of an impermeant solute can drive water into the cell!
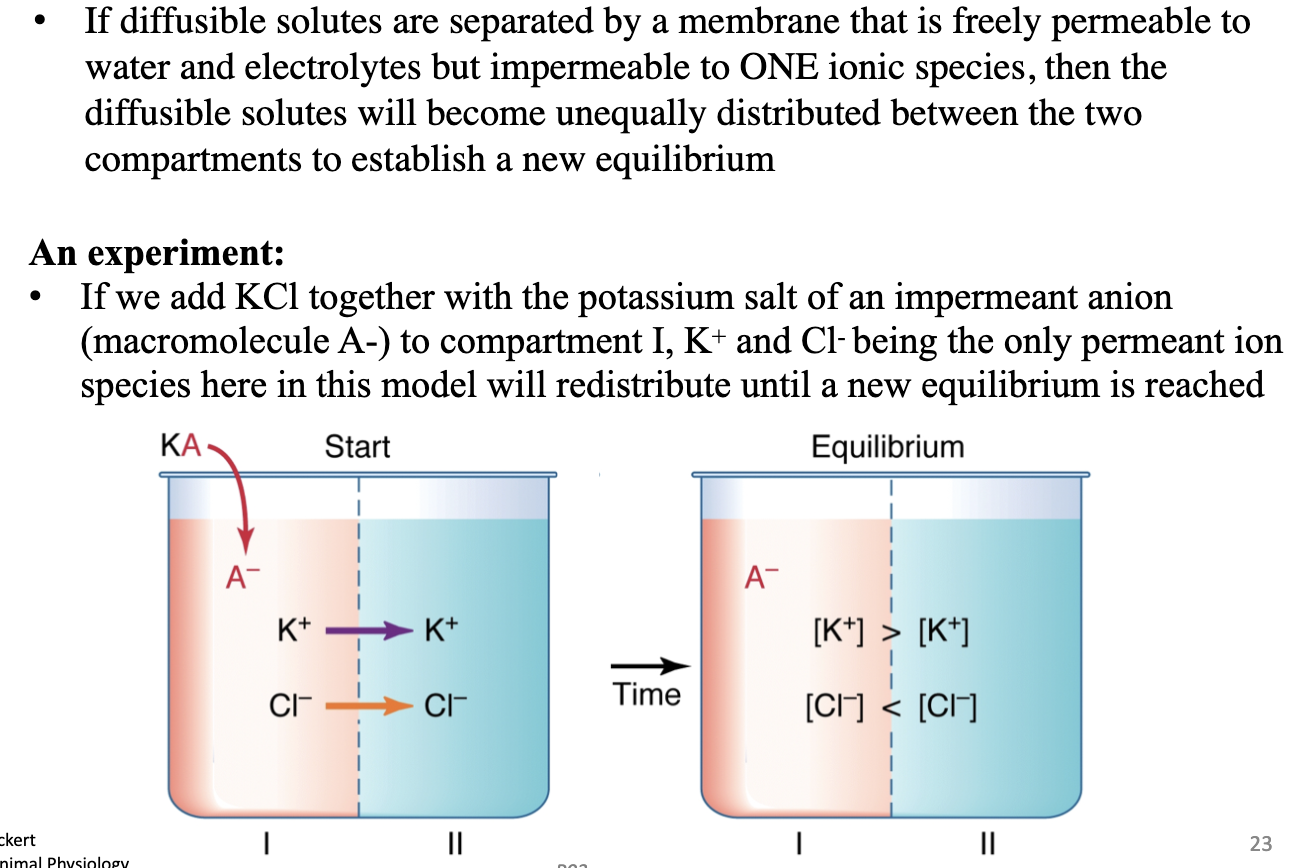
What happens when impermeant ions (A–) are present?
Permeant ions (e.g., K+, Cl–) redistribute unequally to reach a new electrochemical equilibrium → unequal ion distribution.
Gibbs-Donnan equilibrium seen for passive transport
What are the 3 conditions for Gibbs-Donnan equilibrium?
(1) Charge balance (electrical neutrality):
Each side of the membrane (ECF and ICF) must have equal total positive (+) and negative (–) charges.
Example: If the inside has lots of negatively charged A– proteins, it must keep enough positive ions (like K+) to balance them.
(2) Concentration product equality:
For the permeant ions (ions that can cross the membrane, here K+ and Cl–), their concentrations arrange so that:
[K+]in x [Cl−]in = [K+]out x [Cl−]out
This ensures the driving forces for diffusion and electrical attraction/repulsion balance out.
(3) One common membrane potential:
The equilibrium potential for K+ and Cl– ends up being the same.
That shared value is the membrane potential (Em), so:
Em = EK = ECl
This means there’s no net movement of K+ or Cl– anymore.
![<p><strong>(1) Charge balance (electrical neutrality):</strong></p><ul><li><p>Each side of the membrane (ECF and ICF) must have equal total positive (+) and negative (–) charges.</p></li><li><p>Example: If the inside has lots of negatively charged A– proteins, it must keep enough positive ions (like K+) to balance them.</p></li></ul><p></p><p><strong>(2) Concentration product equality:</strong></p><ul><li><p>For the permeant ions (ions that <em>can</em> cross the membrane, here K+ and Cl–), their concentrations arrange so that:</p><ul><li><p>[K+]in x [Cl−]in = [K+]out x [Cl−]out</p></li></ul></li></ul><ul><li><p>This ensures the driving forces for diffusion and electrical attraction/repulsion balance out.</p></li></ul><p></p><p><strong>(3) One common membrane potential:</strong></p><ul><li><p>The equilibrium potential for K+ and Cl– ends up being the same.</p></li><li><p>That shared value is the membrane potential (Em), so:</p><ul><li><p>Em = EK = ECl</p></li></ul></li></ul><ul><li><p>This means there’s no net movement of K+ or Cl– anymore.</p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/0d038854-9237-4004-865a-3677d17049fe.png)
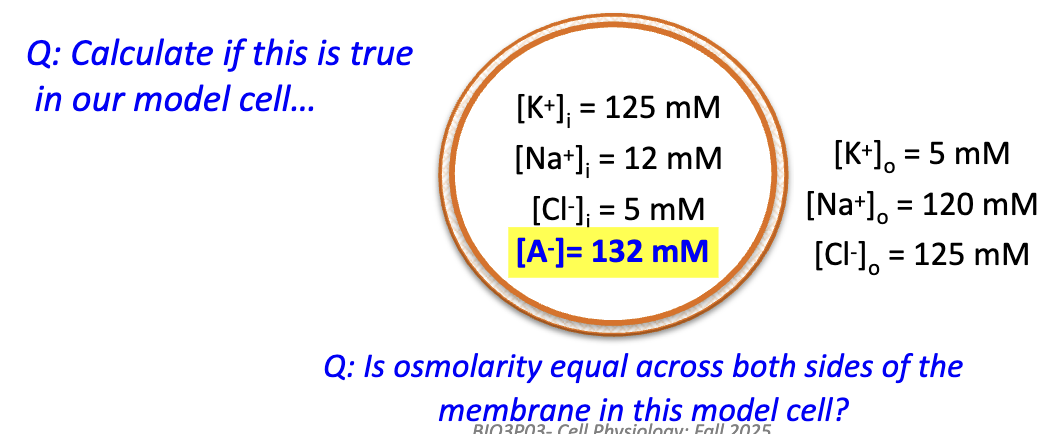
In the model cell with impermeant A–, is osmolarity equal across the membrane?
No → imbalance causes osmotic stress (water tends to enter cell).
This cell is in Gibbs-Donnan equilibrium.
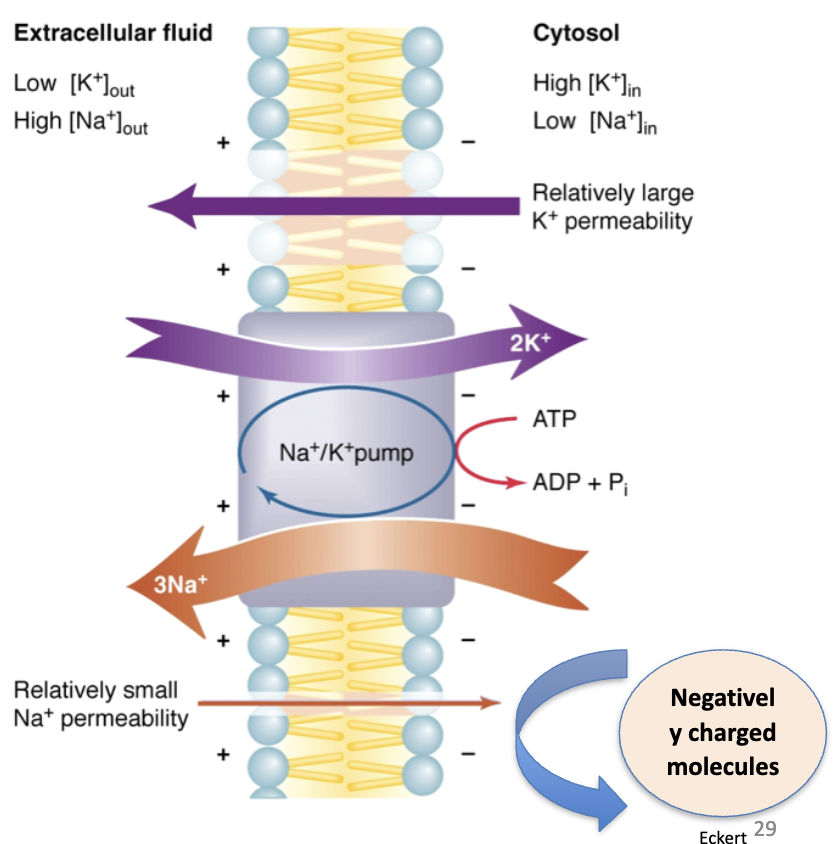
How does the Na⁺/K⁺ pump prevent osmotic catastrophe in cells?
By pumping Na⁺ out (using ATP), it counteracts impermeant anions inside the cell, prevents excess water influx, maintains osmotic equilibrium, and keeps the cell in steady state (no net ion flux at equilibrium).
What real-world case highlights the importance of the Na⁺/K⁺ pump?
1982 Chicago: Tylenol poisonings, where potassium cyanide–laced capsules caused deaths by blocking ATP production, which disabled the Na⁺/K⁺ pump.
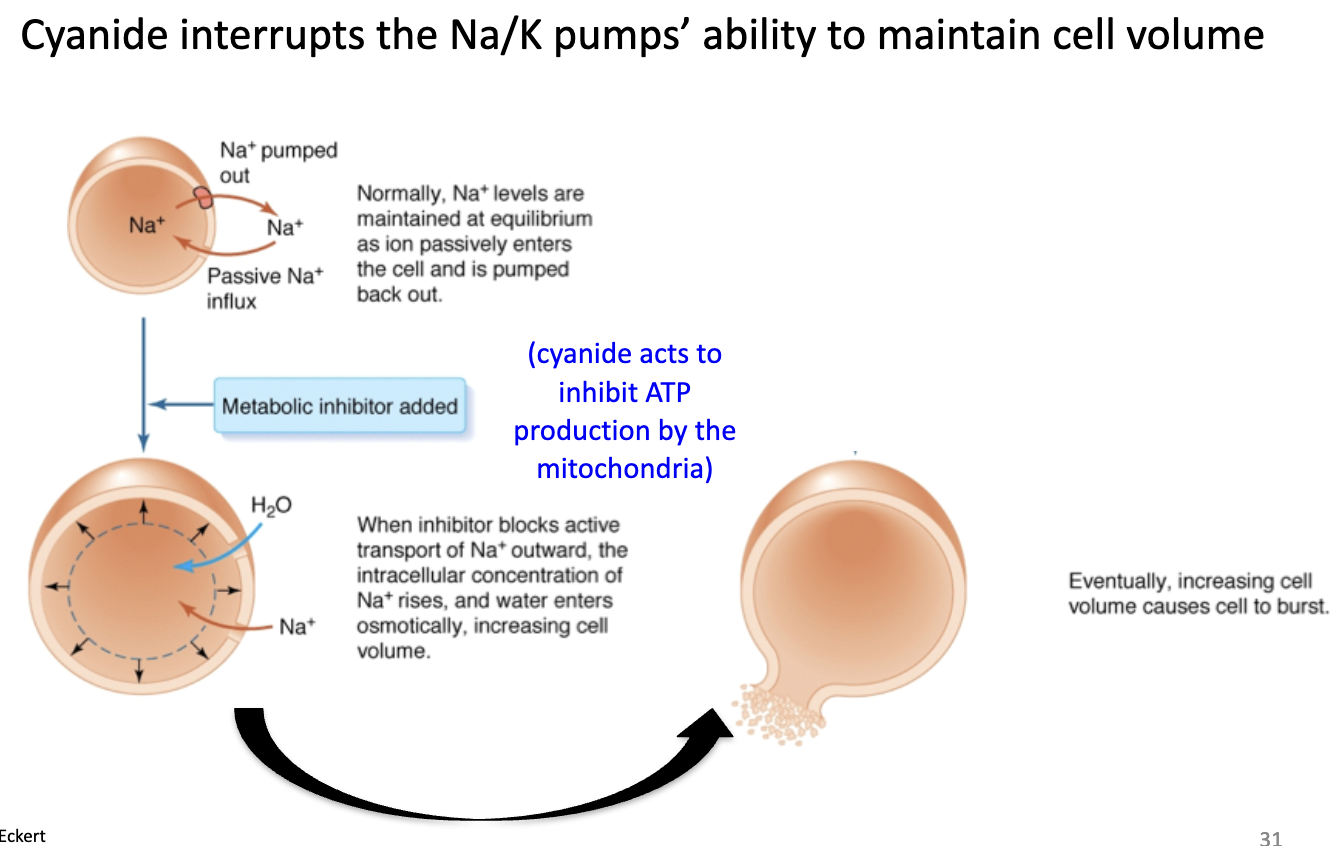
Why is the Na/K pump clinically important (e.g., Tylenol scare)?
Poisoning (cyanide) inhibits mitochondrial ATP production → Na/K pump fails → Na+ accumulates inside → water influx → cell swelling and lysis (death).
What was the outcome of the 1982 Tylenol cyanide deaths?
Led to tamper-proof packaging laws and safety reforms for over-the-counter drugs.
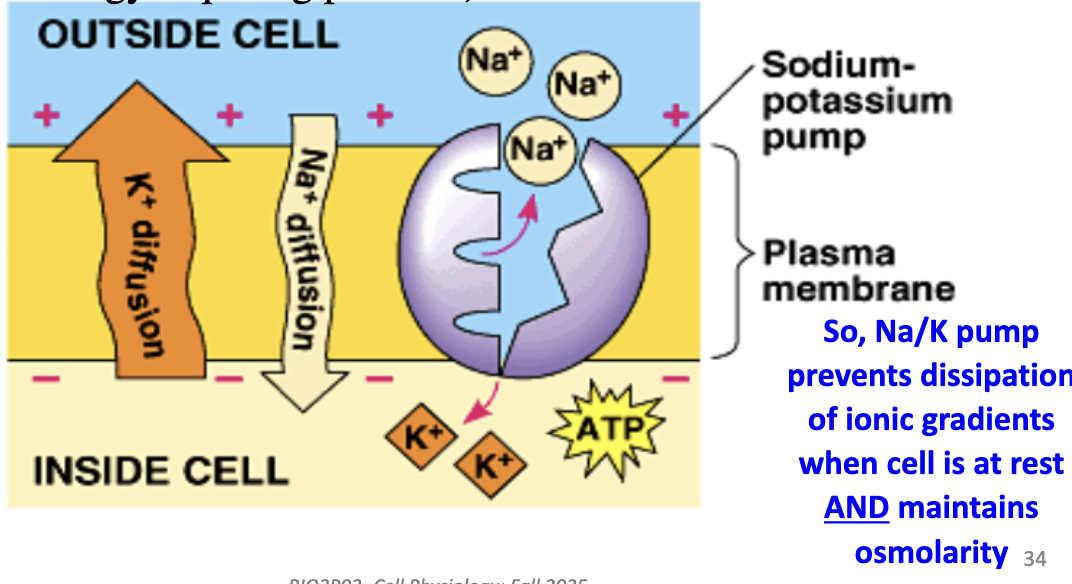
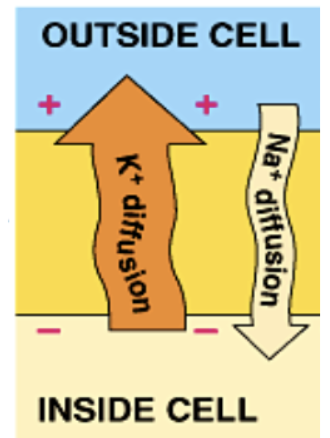
Why doesn’t Vm dissipate due to ion diffusion?
Because Na/K pump actively counters leak of Na+ in and K+ out. Vm is closer to EK since K+ is most permeable.
What are the two main roles of the Na/K pump?
(1) Maintains resting Vm (~ –70 mV) by balancing leaks.
(2) Prevents osmotic swelling by regulating ion gradients, maintaining osmolarity by pumping Na⁺ out and K⁺ in.