BIOL 1020 FINAL Exam
1/199
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
200 Terms
What are the commonalities of all living organisms?
evolution, order/organization, reproduction, growth and development, energy processing, internal regulation, and response to the environment
Evolution
The change in the genetic composition of populations over time, driven by natural selection and adaptation
Order/Organization
The structured arrangement of components in biological systems that enables complex functions and processes essential for life
Reproduction
The biological process by which organisms produce new individuals, ensuring the continuation of their species
Growth and Development
The processes by which organisms increase in size and mature, involving cellular differentiation and specialized functions
Energy Processing
The process by which organisms obtain and utilize energy from their environment to sustain life functions
Internal Regulation
The mechanisms by which organisms maintain stable internal conditions despite external changes, crucial for homeostasis
Response to the environment
The ability of organisms to react and adapt to stimuli in their surroundings, ensuring survival and proper functioning
What is the hierarchy of biological organization?
Biosphere, ecosystems, communities, populations, organisms, organs and organ systems, tissues, cells, organelles, molecules, atoms
What is the classification of biological diversity?
Domain, kingdom, phylum, class, order, family, genus, and species
What are the three domains of life?
Archaea, bacteria, and eukarya
Archaea
Single-celled prokaryotes, differs from bacteria with their cellular structure and biochemistry, can thrive in harsh environment
Bacteria
Single-celled prokaryotes, can be found in a wide range of environments and play important roles in ecosystems
Eukarya
All eukaryotic organisms, including plants, animals, fungi, and protists
Science
A systematic discipline that builds and organizes knowledge in the form of testable hypotheses and predictions about the universe.
Observations
The act of noticing or perceiving something, particularly through the use of one's senses or scientific instruments, and recording or quantifying the information gathered
Hypothesis
A supposition or proposed explanation made on the basis of limited evidence as a starting point for further investigation
What is the scientific method?
Observations, testable hypothesis, controlled experiment, data collection, and conclusions
Theory
A well-substantiated explanation of some aspect of the natural world that is based on a body of facts and has been repeatedly tested and confirmed through observation and experimentation
What is the central theory of biology?
Evolution through natural selection
Matter
Any substances that has mass and takes up space by having volume, (can exist as a solid, liquid, or gas), comprised of elements
Element
A substance which cannot be broken down by chemical menas
Compound
Substance of two or more elements in fixed ratio
Chemical symbols
Elements have chemical symbols consisting of 1 or 2 letter symbols (EX. Au = Gold)
What elements are the most important for life?
Carbon (C), hydrogen (H), nitrogen (N), oxygen (O), phosphorus (P), and sulfer (S)
Why are element’s properties dependent on the structure of its atoms?
The properties of elements depend on atomic structure, specifically the arrangement and number of electrons, protons, and neutrons, which influence chemical behavior and bonding
Chemical bonds
The forces that hold atoms together in molecules and compounds, and include ionic, covalent, and metallic bonds
Strong bonds (intramolecular)
Covalent and Ionic
Weaker bonds (intermolecular)
Hydrogen bonds and Van der Waals interactions
Ionic bonds
Formed by the transfer of electrons from one atom to another, resulting in oppositely charged ions that attract each other
Covalent bonds
Formed when two atoms share electrons, resulting in a stable balance of attractive and repulsive forces between them
Hydrogen bonds
Formed between a hydrogen atom covalently bonded to a highly electronegative atom (life oxygen or nitrogen) and another electronegative atom
Van der Waals bonds
Weak attractions between molecules due to temporary fluctuations in electron distribution
Polar covalent bonding
Unequal sharing of electron pairs between atoms with different electronegativities, leading to partial positive and negative charges
What are the four emergent properties of water?
Cohesion, adhesion, high specific heat, and universal solvent
Acids and bases
Substances that can donate protons (H+) or accept protons, respectively, affecting the pH of solutions
Vitalism
Living molecules only come from living sources; there is a “living" force” found only within organisms that are alive
Mechanism
Living chemistry is complex, but reproducible; molecules from living organisms can be reproduced from non-living sources
Carbon compounds
Organic molecules primarily made of carbon that are essential for life, forming the basis of biological structures and functions
Hydrocarbons Chains
Organic molecules consisting of only carbon and hydrogen; they can undergo reactions that release a large amount of energy
Isomers
Same molecular formula, different structures, and properties
Structural Isomers
Same structural formula, different arrangement of covalent bonds
Cis-Trans Isomers
Vary across a double bond; the two Xs are on the same side
Trans Isomers
The two Xs are on opposite sides
Enatomers
Mirror-image isomers that cannot be superimposed on each other, leading to different chemical properties.
Functional Groups
The components of organic molecules that are most commonly involved in chemical reactions
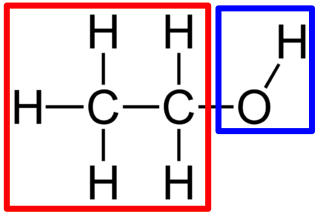
Hydroxyl
Structure: -OH
Functional Properties: Polar and can form hydrogen bonds; increases solubility in water
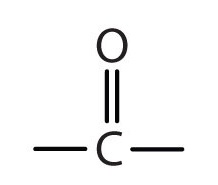
Carbonyl
Structure: C=O; a ketone if the carbonyl group is within a carbon skeleton, a aldehyde if the carbonyl group is at the end of the carbon skeleton
Functional Properties: Polar, involved in reactions such as aldol condensation and ketone formation
Ketones
Acids that your body makes when it breaks down fat for energy
Aldehydes
Organic compounds containing a carbonyl group (C=O) at the end of the carbon chain, which makes them highly reactive
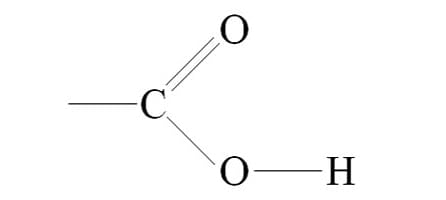
Carboxyl
Structure: COOH
Functional Properties: Acts as an acid; can donate an H+ because the covalent bond between oxygen and hydrogen is so polar; found in cells in the ionixed form with a charge of 1-
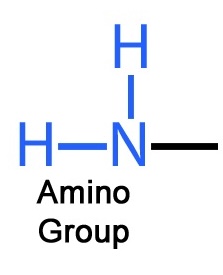
Amino
Structure: -NH2
Functional Properties: Acts as a base; can pick up an H+ from the surrounding solution; found in cells in the ionized form with a charge of 1+
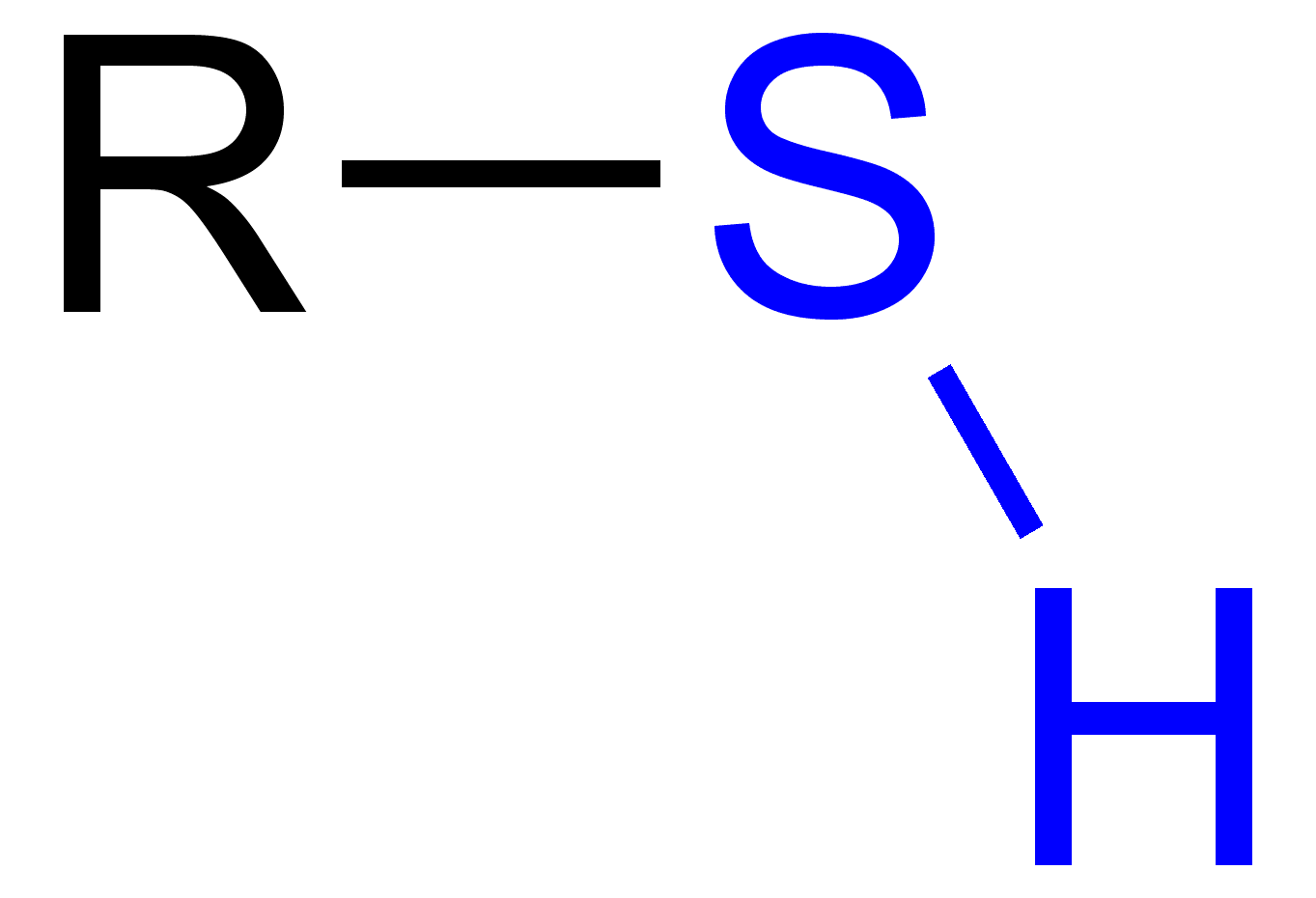
Sulfhydryl
Structure: -SH
Functional Properties: two sulfhydryl groups can react, forming a covalent bond (cross-linking helps stabilize protein structure)
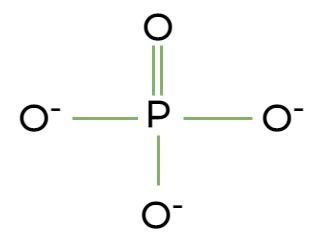
Phosphate
Structure: PO43-
Functional Properties: contributes negative charge to the molecule of which it is a part; molecules containing phosphate groups have the potential to react with water, releasing energy
Methyl
Structure: CH3
Functional Properties: addition of a methyl group to DNA, or to molecules bound to DNA
Macromolecules
Large molecules and are complex
What are the four molecules of life?
Carbohydrates, lipids, proteins, and nucleic acids
Polymer
(many parts) A long molecule consisting of many similar building blocks
Monomers
(single parts) The repeating units that serve as building blocks
Dimers
(two parts) Molecules formed by the combination of two monomers
Trimers
(three parts) Molecules formed by the combination of three monomers
Tetramers
(four parts) Molecules formed by the combination of four monomers
Oligomers
(several parts) Molecules formed by the combination of multiple monomers, typically ranging from 2 to 10.
Which three out of four are polymers in the molecules of life?
Carbohydrates, proteins, and nucleic acids
Enzymes
Speed up chemical reactions such as those that make or break down polymers; made up of protein
Dehydration reaction
Occurs when two monomers bond together through the loss of a water molecule
Hydrolysis
The process of breaking down polymers into monomers by adding a water molecule; it is the reverse of a dehydration reaction
Carbohydrates
Organic molecules consisting of carbon, hydrogen, and oxygen, often serving as a primary energy source and structural components in cells
Monosaccharides
Simple sugars that are the building blocks of carbohydrates
Monosaccharides
are the simplest form of carbohydrates, consisting of single sugar molecules such as glucose and fructose; they are absorbed directly into the bloodstream and provide quick energy.
Disaccharide
Forms when a dehydration reaction joins two monosaccharides; they form a bond called glycosidic linkage
Polysaccharides
The polymers of sugar, have storage and structural roles; structure and function are determined by its sugar monomers and the positions of its glycosidic linkages
Starch
A storage polysaccharide of plants, consists entirely of glucose monomers
Cellulose
A polysaccharide that is a major component of the tough wall of plant cells
Lipids
A diverse group of hydrophobic organic molecules, including fats, phospholipids, and steroids, that play roles in energy storage, cell structure, and signaling
Fats
Constructed from two types of smaller molecules: glycerol and fatty acids
Fatty Acid
Consist of a carboxyl group attached to a long carbon skeleton
Types of Fatty Acids
Saturated fatty acids: the maximum number of hydrogen atoms possible and no double bonds
Unsaturated fatty acids: one or more double bonds
Fats with saturated fatty acids
Have saturated fats, solid at room temperature, includes most animal fats
Fats with unsaturated fatty acids
Have unsaturated fats or oils, liquid at room temperature, in plants and fishes
Hydrogenation
The process of adding hydrogen to unsaturated fatty acids to convert them into saturated fats, often used in the food industry to improve shelf stability
Omega-3 fatty acids
Certain unsaturated fatty acids are not synthesized in the human body and must be obtained from the diet.
These essential fatty acids include the omega-3 fatty acids; these acids are required for normal growth
Phospholipids
A class of lipids that are a major component of all cell membranes, consisting of two fatty acids and a phosphate group attached to a glycerol backbone
Steroids
Lipids characterized by a carbon skeleton consisting of four fused rings with various functional groups attached, involved in a variety of biological processes including hormone regulation
Cholesterol
A type of steroid that is a key component of cell membranes and serves as a precursor for the synthesis of steroid hormones and bile acids
Proteins
Include a diversity of structures, resulting in a wide range of functions; accounts for more than 50% of the dry mass of most cells
What are some protein functions?
Proteins serve as enzymes, structural components, transport molecules, antibodies, and hormones, playing vital roles in cellular processes and functions.
Enzymes
Speed up chemical reactions such as those that make or break down polymers; made up of proteins
Storage proteins
Function to store amino acids and other nutrients for later use, playing a critical role in energy and resource management within organisms
Example: Casein, the protein of milk, is the major source of amino acids for baby mammals, plants via their seeds, and ovalbumin, the protein in egg whites, is used as an amino acid source
Hormonal proteins
Coordination of an organism’s activities
Example: Insulin, a hormone secreted by the pancreas, causes other tissues to take up glucose, regulating blood sugar concentration
Contractile and motor proteins
Function: Movement
Examples: Motor proteins are responosible for the undulations of cilia and flagella; actin and myosin proteins are responsible for the contraction of muscles
Defensive proteins
Function: Protection against disease
Example: Antibodies inactivate and help destroy viruses and bacteria
Transport proteins
Function: Transport of substances
Example: Hemoglobin, the iron-containing protein of vertebrate blood, transports oxygen from the lungs to other parts of the body
Receptor proteins
Function: Response of cell to chemical stimuli
Example: Receptors built into the membrance of nerve cell detect signaling molecules released by other nerve cells
Structural proteins
Function: support
Examples: Keratin is the protein of hair, horns, feathers, and other skin appendages; insects and spiders use silk fibers to make their cocoons and webs, respectively
Polypeptides
Unbranched polymers built from the same set of 20 amino acids
Protein
Biologically functional molecule that consists of one or more polypeptides
Amino acids
Organic molecules with carboxyl and amino groups
Differ in their properties due to differing side chains, called R groups
What are amino acids are linked with?
Peptide bonds
What does a functional protein consist of?
One or more polypeptides precisely twisted, folded, and coiled into a unique shape