How fast? rate of reaction
1/28
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
29 Terms
rate of reaction
speed at which a chemical reaction takes place, can be expressed as change in conc of a particular reactant/product per unit time
calculating rate of reaction (mol dm-3 s-1 )
change in conc of reactants or products / time
3 techniques of measuring rate
mass loss
gas production
colorimetry
4 factors of collision theory
for a reaction between 2 particles to occur, the particles must collide with:
the appropriate collision geometry
sufficient kinetic energy
the minimum amount of energy required = the activation energy
factors affecting rate of reaction
concentration
pressure
temperature
SA
catalysts
concentration affecting rate
an increase in conc causes an increase in number of particles per unit volume
this causes increase in collision frequency, hence increased frequency of successful collisions
pressure affecting rate
increase in pressure causes less space for particles to move in
number of successful collisions increases due to increased collision frequency
temperature affecting rate
increase in temp cause particles to move faster as they gain KE, so collide more frequently
at higher temp, higher proportion of particles have the activation energy, meaning higher proportion of collisions are successful
surface area affecting rate
increase in surface area means more particles are on the surface and able to collide with particles of reactant
means more collisions in total meaning more successful collisions
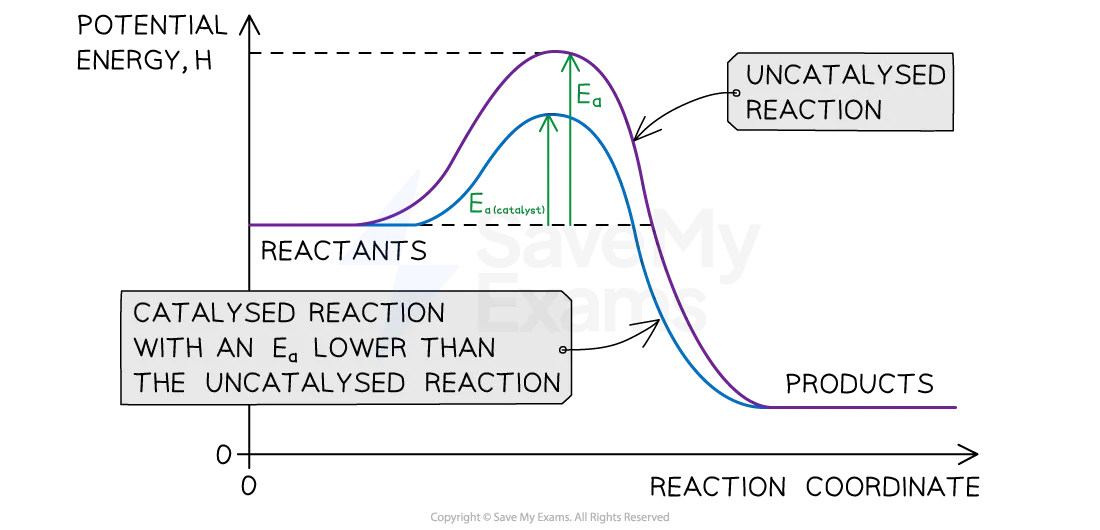
catalyst affecting rate
catalyst provides reactants with alternative reaction pathway which is lower in activation energy
more collisions are successful
catalyst remains unchanged
activation energy
minimum energy colliding particles need to collide successfully leading to a reaction
homogenous & heterogenous catalyst
homo: catalyst is in the same phase as reactant
hetero: catalyst is in a different phase to the reactant
energy profiles of catalyst reactions
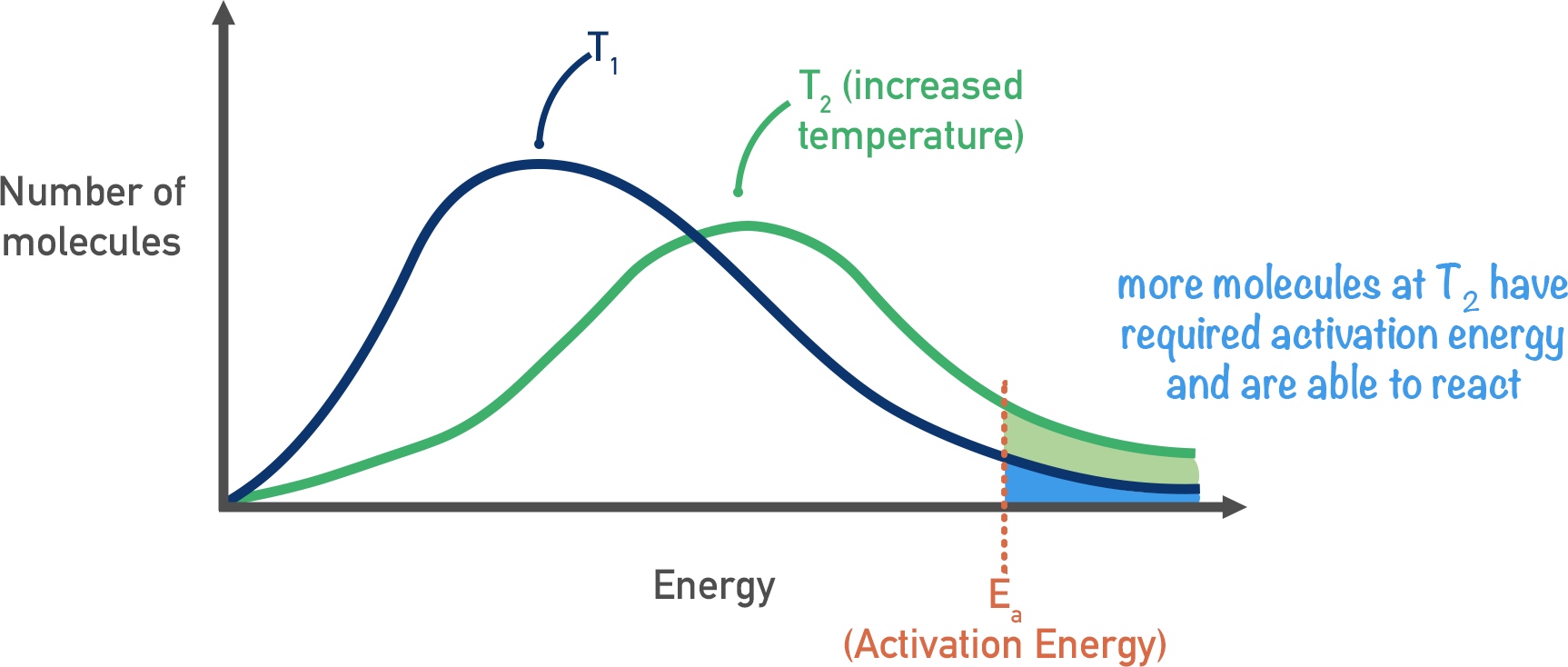
effect of temp on maxwell boltzmann
when temp increases, curve flattens and the peak shifts to right
because higher proportion of successful collisions
rate equation
Rate of reaction = k [A]m [B]n
A and B are concs of reatants
catalysts and products may feature
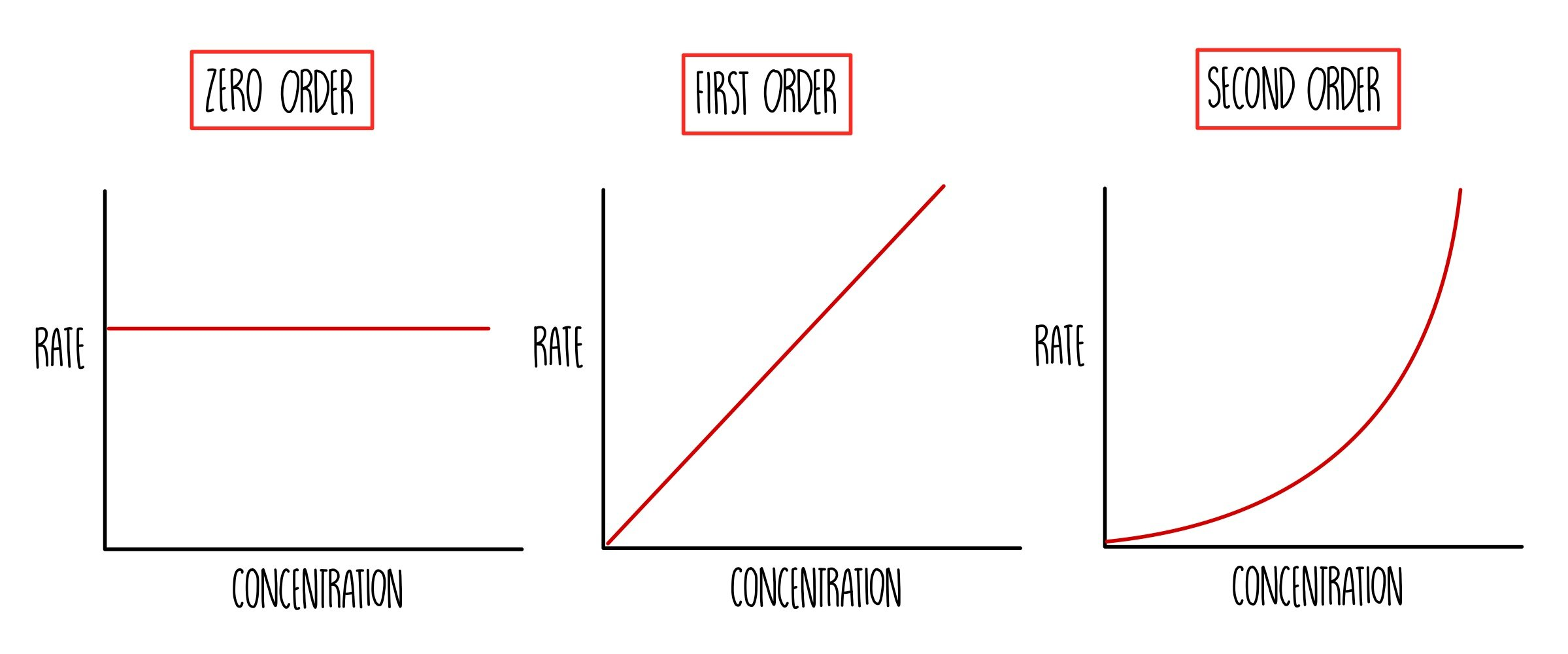
order of reaction
order shows how conc affects the rate
the power to which the conc of the reactant is raised in the rate eqution
zero order
changing conc has no effect
first order
conc of reactant is directly proportional to rate
second order
rate is directly proportional to the square of conc of reactant
determining rate equation from data
find 2 experiments where conc of 1 reactant changes but concs of others are constant
calculate what happens to concentration and rate
deduce order
reaction orders from conc-time graphs
zero order
as time goes on, conc decreases, graph is straight line
rate = k
first order
conc decreases with time, graph is a curve which plateaus
second order
conc decreases more steeply with time, graph is steeper curve which plateaus
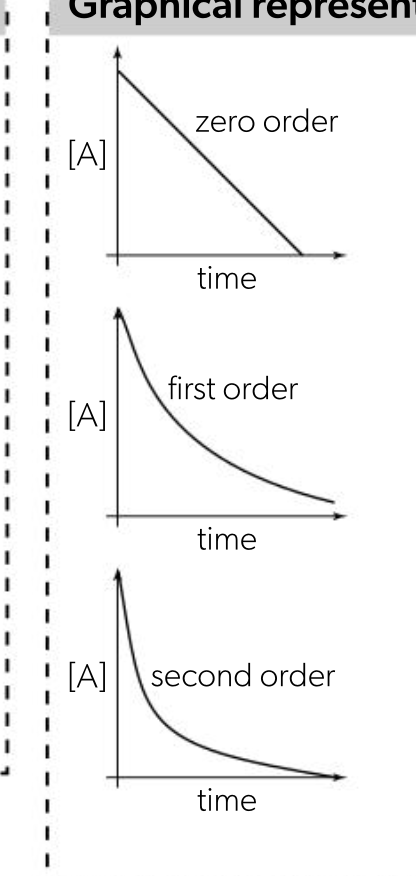
reaction orders from rate-conc graphs
zero order
rate remains constant, graph is horizontal line
rate = k
first order
rate increases as conc increases,
graph is straight line
rate = k(A)
second order
rate increases more as conc increases, graph is curved line
rate = k(A)2
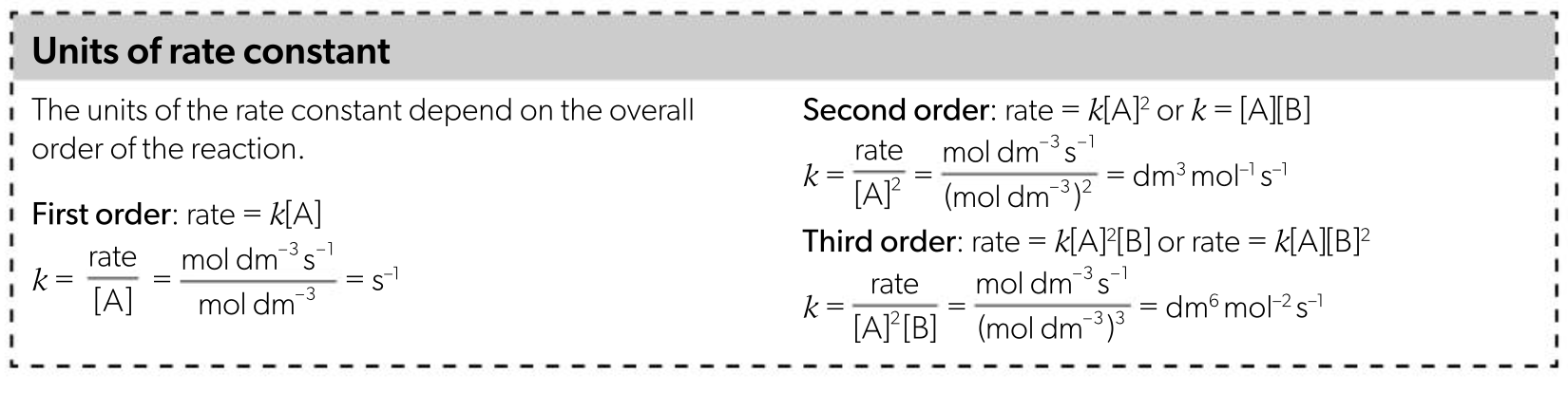
calculating the rate constant and its units
zero order: mol dm-3 s-1
effect of temp on rate constant
increasing temp increases value of rate constant as concentration stays same
this is because as rate of reaction increases, rate constant increases
relationship is not linear though
reaction mechanisms
each step in reaction is called elementary step, involves small number of particles
some products of an elementary step exist as intermediates and react in subsequent steps
sum of elementary steps must equal overall reaction equation
intermediates cancel out if appear on both sides
rate determining step
the slowest step in the reaction
if reactant appears in rate determining step, conc of the reactant appears in rate equation
the order with respect to a reactant is the number of particles of that reactant that take part in rate-determining step
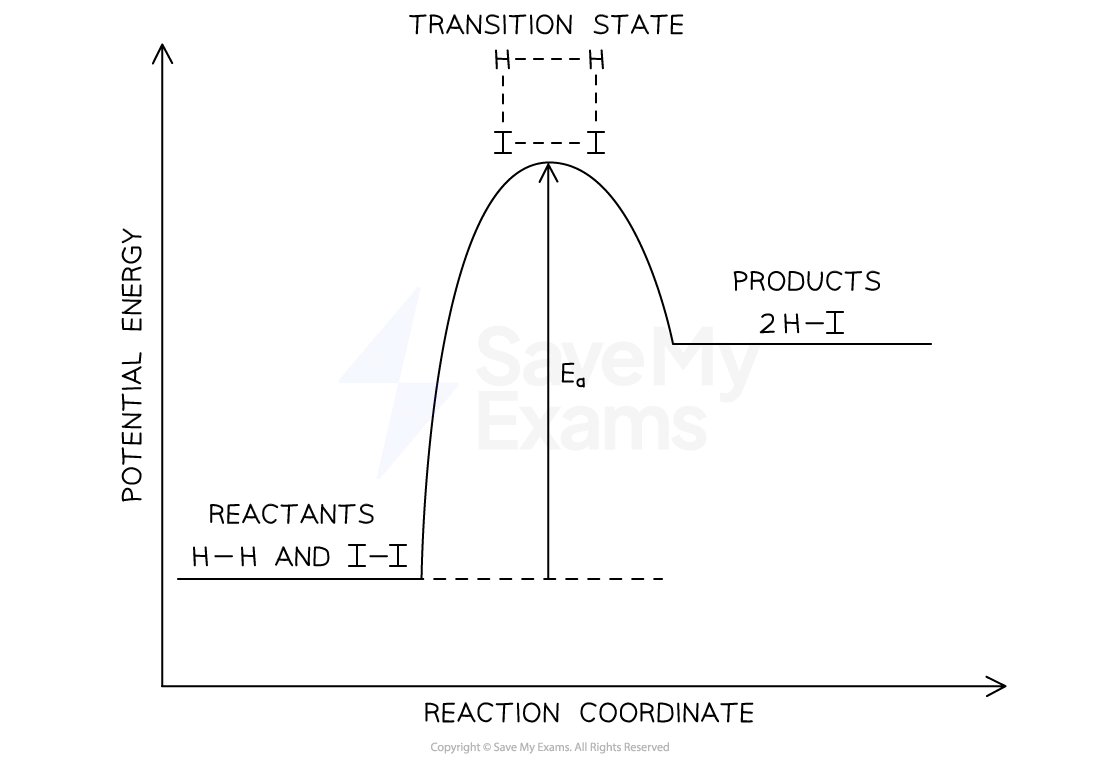
energy profiles in single step reaction
when reacting molecules collide, with bond breaking/formation occurring, they will be in an unstable, high-energy state temporarily
transition state: higher energy state than reactants/products, corresponds to AE
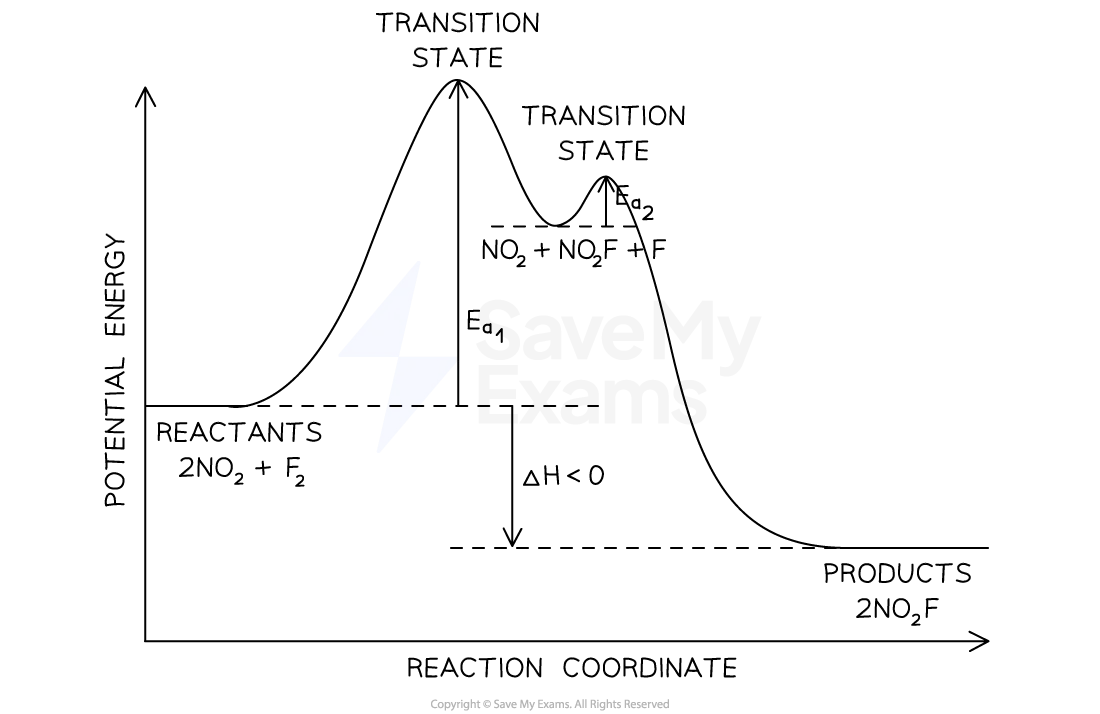
energy profiles in multi step reactions
the rate determining step is the step with greatest activation energy
molecularity
number of reacting particles taking part in an elementary step
unimolecular: one reactant particle involved
bimolecular: two reactant particles involved
termolecular: three reactant particle involved
usually discussed in relation to the rate determining step
arrhenius equation
k = rate constant
A = arrhenius factor (nature of reactants)
Ea= activation energy J mol-1
R= gas constant
T= temperature K
k and T are the only variables
used to describe reactions involving gases, occurring in solution, or reactions on surface of catalyst
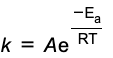
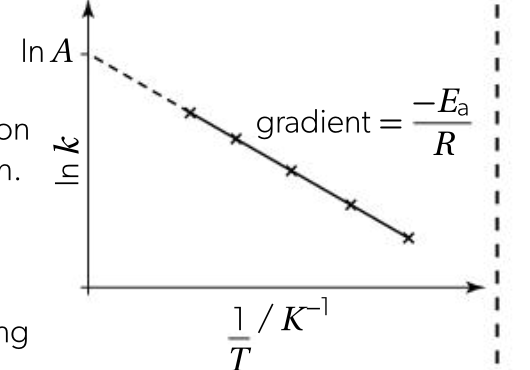
using the arrhenius equation
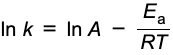
determining Ea and the arrhenius factor
once k at different temperatures for a reaction has been determined, can be used to determine Ea and A
graph of ln k against 1/T can be plotted
gradient: -Ea / R
y intercept: ln A
Ea = -gradient x R
A = ey-intercept