ANSC 424- 5 Cardiovascular and Adrenal Medulla
1/93
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
94 Terms
what is blood osmolality?
Solute concentrations
same as osmolarity BUT just different units Osmolarity = Osmol/L; Osmolality = Osmol/Kg ( osmoloaity is better because kg NOT temp dependent)
what is a normal blood osmolality
Normal blood osmolality: 280 to 295 mOsm/kg
what recognizes changes in osmolality
Osmoreceptors
where can Osmoreceptors be found
Osmoreceptors located in OVLT and SFO(hypothalamus) detect osmotic changes in blood
what are osmoreceptors sensitive to
Osmoreceptors most sensitive to sodium (Na+)
what is blood pressure sensed by
baroreceptors located in aortic arch and carotid sinus
what are baroreceptors structurally
Baroreceptors are not a single protein, but a complex of mechanosensitive ion channels
what genes can encode for baroreceptors
Candidate genes include ASIC2, Peizo 1 and 2, and ENaC (DEG/ENaC family proteins)
what is the major determinant of blood pressure in arteries
diameter of arterioles and control the distrubition of blood supply to tissues
Anatomy of nephron?
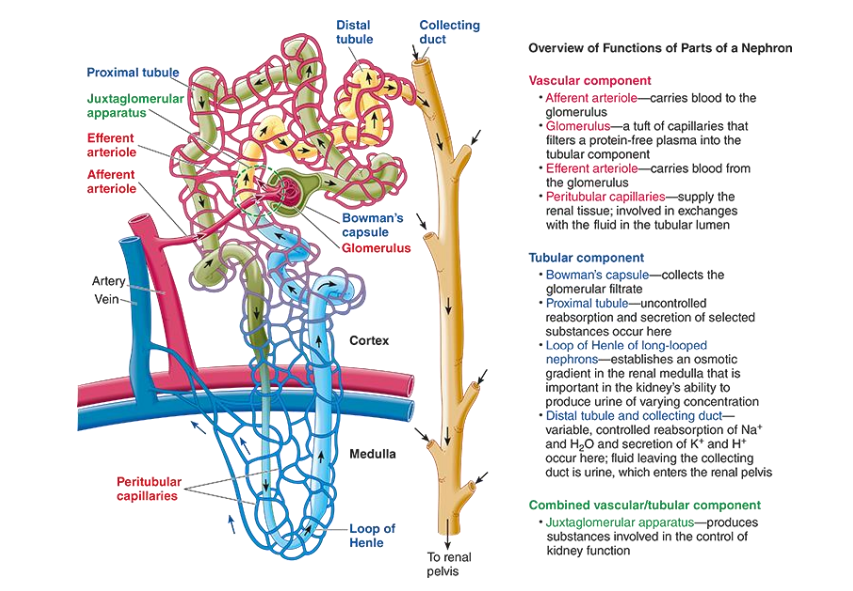
what are the main hormones responsible in the Regulation of blood volume, pressure and osmolality
Regulated by multiple hormonal mechanisms
❖Vasopressin
❖Renin-Angiotensin-Aldosterone
❖Natriuretic peptides
where does vasopressin come from
Hypothalamic nuclei with magnicellular neurons from SON
—> FROM PSOTERIOR PITUITARY GLAND
what is the main functions of vasopressin
❖ H2O retention by the kidney
❖ Contraction of blood vessels (arterioles)
❖ Involved in regulation of the cardiovascular system
what are the different receptors for vasopressin?
V1 ( a and B) and V2
where are V1a found and function
Target cell: Smooth muscles, Platelets, Hepatocytes
Function: Vasoconstriction, platelet aggregation, Glycogenolysis
where is V1b found and function
Target: Anterior pituitary
Functions: ACTH release
what is the function of V2 and where is it found
Target cell: Collecting tubule (kidney) Endothelium
Functions: AQP2 synthesis and translocation vWF and Factor 8 release
what can cause vasoconstrictrion?
increase myogenic atcivity, oxygen, sympathetic stimulation, vasopressin, angiotensin II
decrased Co2
what can cause vasodilation
decreased myogenic activity, O2, sympathteic stimulation, histamine, heat
increased Co2
explain the vasopressin receptor signaling
explain how vasopressin can correct low bp
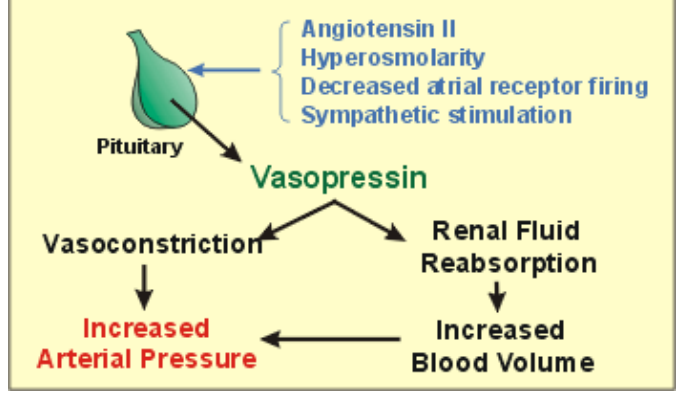
what is relationship of vasopressin and water loss
Vasopressin can bring water loss through urine production to minimum but cannot stop it ❖Insensible water loss – respiration and perspiration
—> small water loss
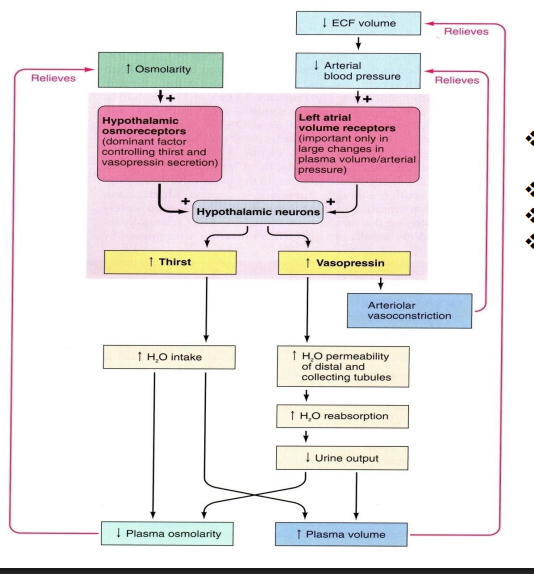
what triggers thirst
Triggered by changes in osmolality or volume, Strongly triggered by hypovolemia and decrease in blood pressure
—> Mechanisms are similar to vasopressin secretion
explain the process by vasopressin leads to thirst
ethanol depresses the hypothalamic osmoreceptor
less vasopressin
increased urine output
dehydration
vasopressin and pregnancy?
—> the brain becomes less sensitive, so it allows for more water retention before it signals the need to adjust
—> increased in blood volume
—> Blood vessels dilate (widen) during pregnancy, so the body adjusts and thinks this new, expanded volume is normal.
—> not as mush vasopressin secretion, rather ajustst to a nre threshold
—> the placenta makes an enzyme that breaks down vasopressin BUT if there is too much expression of this can lead to gestational diabetes insipidus
vasopressin and the elderly?
—> By age 80, total body water declines to as low as 50%
—> The kidneys become less efficient at filtering blood, which affects water and salt balance
—> collecting duct in kidney less responsive to vasopressin, harder to retain water
—> brain cant really detect dehydration real well
—> elderly susceptible to both hypo and hypernatremia
what is diabetes insipidus
excretion of a large volume of urine (diabetes) that is hypotonic, dilute and tasteless (insipid)
what are the causes of diabetes insipidus
❖ lack of vasopressin (trauma, tumour etc)
❖ lack of response to vasopressin in kidney
❖ receptor defect or aquaporin defect
❖ Rapid metabolism of vasopressin
❖ Pregnancy i.e. transient diabetes insipidus
what are charcteristic features of people with diabetes inspidus
polydipsia and polyurea
what is polydispsia
people that drink TOO much water
what is polyurea?
people that urinate too much
what are the main functions of aldosterone
❖ Recovery of Na+ in the kidney and enhanced K+ secretion into the urine to balance charge difference
❖Adjustment of extra-cellular fluid (ECF), including blood volume
—> retain sodium and excrete K
pathway of aldosterone synthesis?
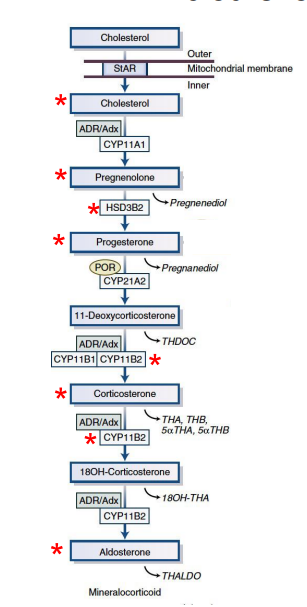
where does aldosterone synthesis occur
zona glomerulosa
where is renin produced
kidneys!
main function of renin
Renin is a hormone/enzyme made by the kidney to help regulate blood pressure and fluid balance. I
explain how renin is produced?
—> produced when the kidney senses low blood pressure or low sodium.
Macula densa cells detect Na+ levels in kidney tubule
Juxtagolomerular cells of afferent arterioles detect blood pressure
Pericytes (not shown) near afferent arterioles produce renin
what does renin do
Renin from pericytes in kidney converts angiotensinogen to angiotensin I in the liver
explain the Renin-angiotensin-aldosterone system
renin convert angiotensinogen—> angiotensin I in liver
ACE from endothelial cells of lungs converts angiotensin I to angiotensin II
angiotensin II stimulates aldosterone secretion (ACTH has a modest effect)
Aldosterone and Angiotensin II increase Na+ absorption and K+ excretion, water retention in kidney
how is angiotensin II inactivated?
Angiotensin II is inactivated to angiotensin III by Aminopeptidase A
Where Does Aldosterone Act?
tubular epithelium through nuclear receptors
what receptor does aldosterone act on
mineralcorticoid receptor ( MR)
—> found in cytoplasm and then acts as TF in nucleus
What Does Aldosterone Do in Epithelial Tissues?
❖ Regulation of fluid volume
❖ Water absorption
❖ Sodium/potassium homeostasis
❖Na+ transport in distal tubules of kidney, colon, Salivary and Sweat glands
—> basically sodium balance
what specific channel is important in aldosterone pathway
ENac
sodium comes in and therefore water also comes in —> need water excretion with aquaporin through vasopressin signaling
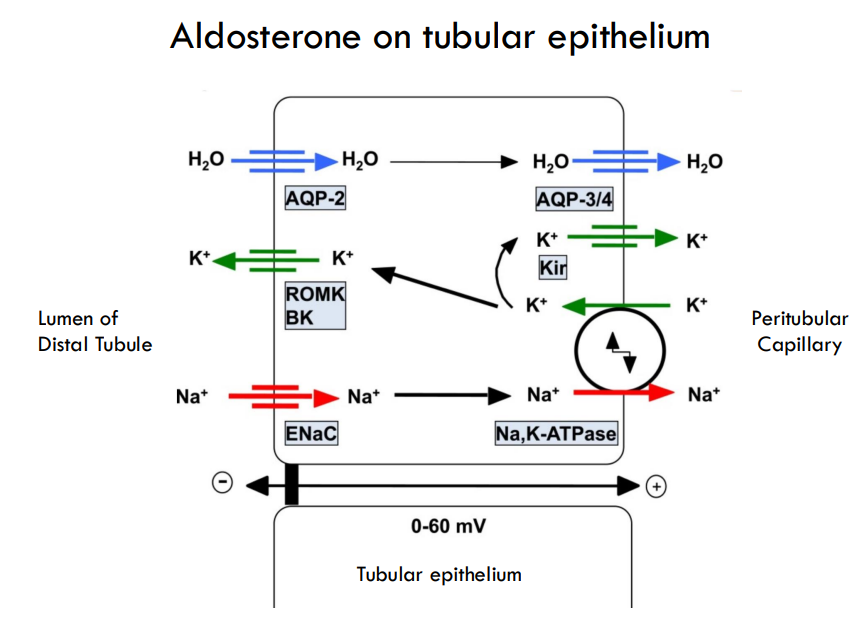
what are the main effects of aldosterone
Mainly: Distal tubules and collecting ducts of the kidney.
Promotes plasma retention of Na+ and excretion of K+
Sensitizes arterioles to vasoconstrictor agents, AVP and Angiotensin II
Net effect: Rise in plasma volume and blood pressure
what is Hyperaldosteronism known as
Conn’s disease
what is the usually cause of Hyperaldosteronism
Hypersecretion of aldosterone usually caused by adrenal hyperplasia (60 %) or tumor (40%)
what are the effects of Hyperaldosteronism
❖Excess excretion of K+ and H+
❖Serum alkalosis and neuropathy (hypokalemia)
❖Increased Na reabsorption
❖Increased water retention
❖Increased blood pressure
—> excess K and H so increased pH—> alklosis and neuropathy
what is Hypoaldosteronism known as
Addison’s disease
what are the causes of addisons
❖ Shortage (deficiency) aldosterone production
❖ Impaired function of aldosterone
what are the symptoms of addison’s
❖ Symptoms:
❖ Low sodium (hyponatremia)
❖ Too much potassium (hyperkalemia)
❖ Low blood pressure
❖ Metabolic acidosis
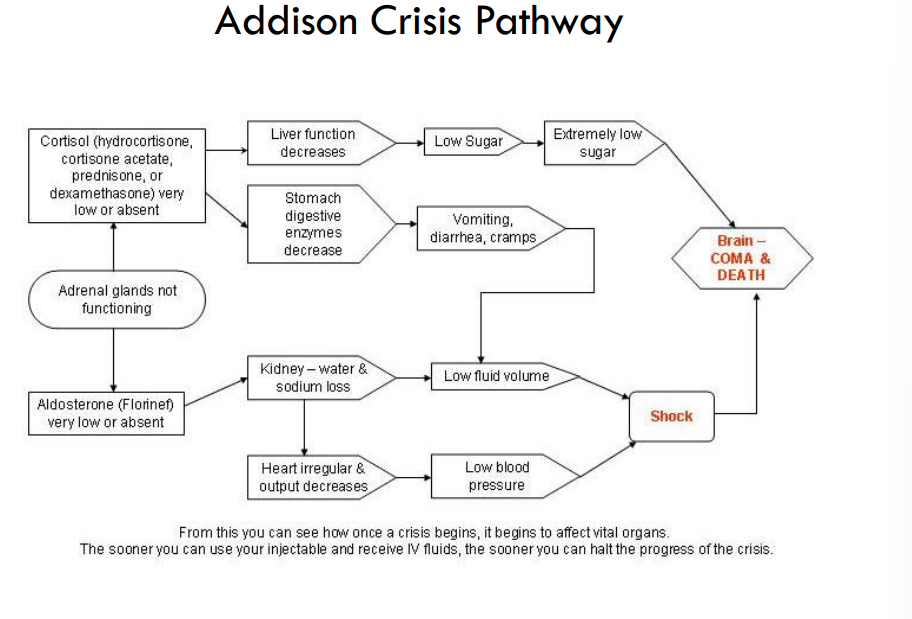
explain addison crisis pathway
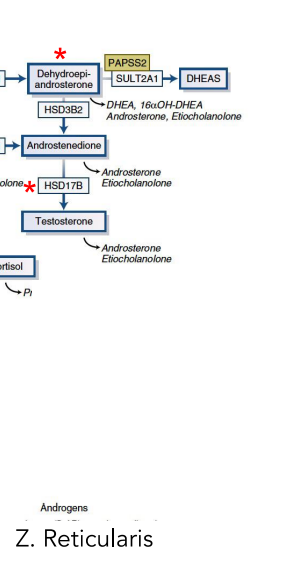
what happens in zona reticularis
andorgen synthesis
explain the androgen synthesis
where are sex steriods usually synthesized
gonads
F: estrogen and progesterone
M: androgens
what regulates sex steriods syntehsis
gonadotrophins
what is the MAIN regulator of aldosterone
angiotensin II
what does the adrenal cortex ( zona reticularis) mainly produce
DHEAS and androstenedione regulated by ACTH
where is androgen—> testesterone
periopheral tissues
what is Congenital adrenal hyperplasia (CAH)
condition in which adrenal glands are enlarged and hyperfunctioning
what causes CAH
mainly genetic due to involves deficiency of CYP21A2 (ZG and ZF)
what happens in CAH
Reduced aldosterone and cortisol
what alternative pathway can be used in CAH
But ZF, with produces 21-deoxycortisol – similar to cortisol but lower glucocorticoid activity
what happens in CAh due to low aldosterone
Low aldosterone leads to Salt wasting, salt and water craving, vomiting and dehydration, low blood pressure (death)
what is highly expressed in CAH
Excessive androgen production in ZR
❖ Masculinization of genitalia
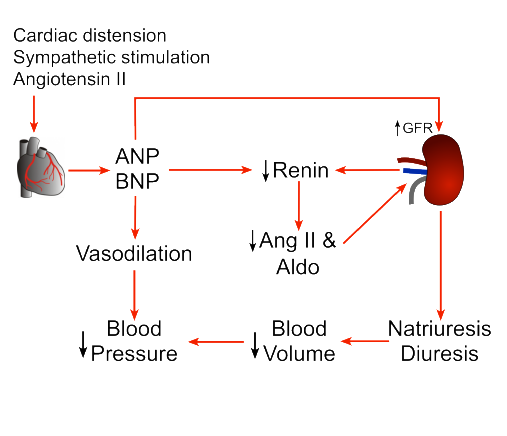
what do Natriuretic peptides do
NP function: Peptides that increase the excretion of H2O and Na+
where can natruitetic peptides found
Produced in the heart muscle cells and stored in granules
where are NP receptors found
Receptors are present in the glomeruli, medullary collecting ducts of the kidney, the zona glomerulosa of the adrenal cortex and in peripheral arterioles
what is the effect of NP
❖ Increases glomerular filtration
\❖ Reduce renin and inhibit aldosterone production
❖ increase water loss (diuresis) and vasodilation
❖ Reduces blood volume and pressure
pathway of NP
what kind of cells can be found in adrenal medulla
modified post ganglionic neurons—> known as chromaffin cells
PART OF SNS
what do Preganglionic neurons release
acetylcholine to stimulate chromaffin cells to release catecholamines
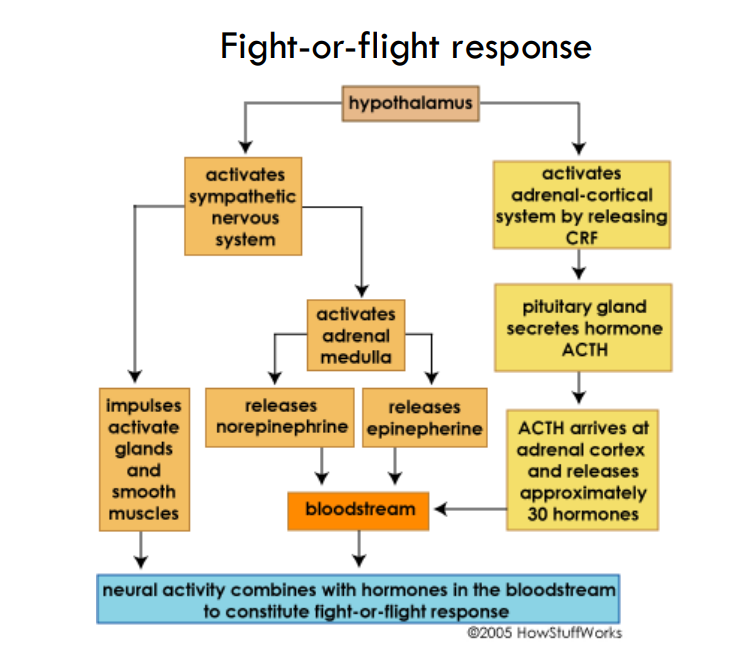
what is the overall effect of catecholamines
Coordinate fight-flight response to alarm by increasing blood pressure and cardiac output, and dilating pupils
what are catecholamines derivatives of
tyrosine
what are examples of catecholamines
norepineprhine and epunephrine
pathway of catecholime synethesis?
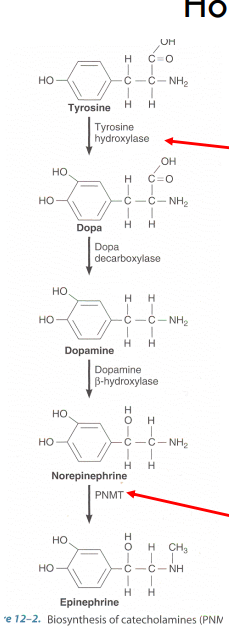
what is the rate limiting step of catecholamine syn?
tyrosine hydroxylase
what is PNMT
Phenylethanolamine Nmethyltransferase (PNMT)
converts norepi—> epi
stimulated by CORTISOL
when are catehcolamines released
in response to STIMULI
—> 80% epi and 20% norepi
explain fight or flight response
release CRF—> ACTH
activate SNS—> release epi
—>
what receptor with EPI and NORepi
GPCR
what does epineprhine do
❖ rapidly mobilizes fatty acids as the primary fuel for muscle action
❖ increases muscle glycogenolysis
❖ mobilizes glucose for the brain by increase hepatic glycogenolysis and gluconeogenesis
❖ preserves glucose for CNS by dcrease insulin release leading to reduced glucose uptake by muscle/ adipose (opposes Insulin)
❖ Increases cardiac output
what does norepinephrine do
increase blood flow and decrease insulin secretion
what receptors for epi and norepi
adregenergic receptors:
Alpha 1 and 2
Beta 1
Beta 2
what does α1/2 and β1 receptors bind to
epi and norepi
what does B2 receptor bind to
mostly epi
how can different responses happen with epi and norepi
tissue specific receptors so you have a different response due to different concentrations of epi and norepi
—> use in drugs
what is Salbutamol
drug that takes adv that epi and norepi receptors are tissue specific
activates β2 receptors and dilates bronchioles (relief of asthma) and does not affect β1 receptors in the heart
what are the different adregenic receptors and target tissues
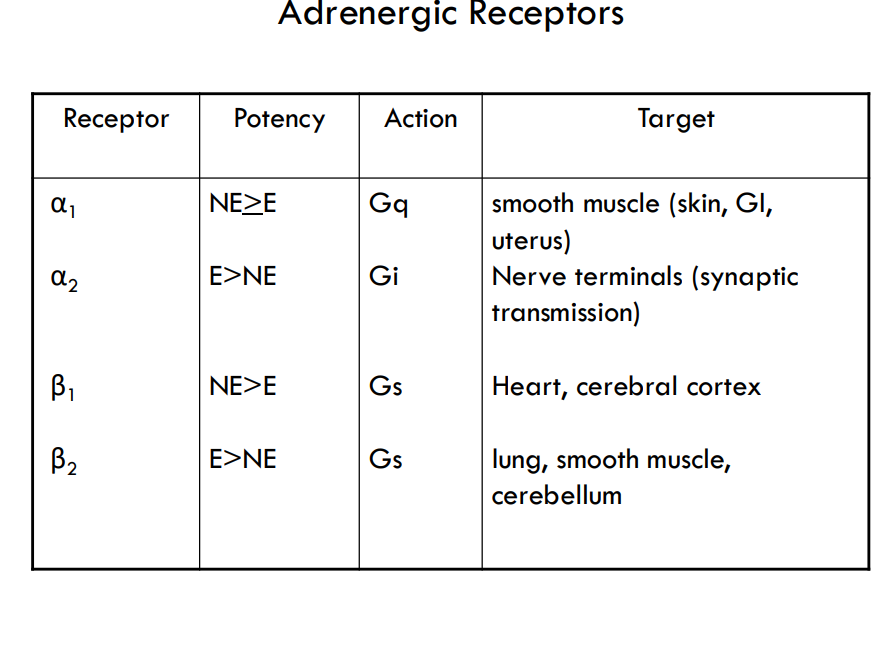
what are the difference between epi and norpepi
cardiac stimulation
—> Epinephrine >> norepinephrine – in terms of cardiac stimulation leading to greater cardiac output (beta1 stimulation)
constriction of blood vessels
—> Epinephrine < norepinephrine – in terms of constriction of blood vessels – leading to increased peripheral resistance – increased arterial pressure
increasing metabolism
—> Epinephrine >> norepinephrine – in terms of increasing metabolism
how can Adrenomedullary deficiency occur
can occur due to surgery/trauma
—> cause cortisol suppression (epinephrine deficiency)
what are symptoms of Adrenomedullary deficiency
Hypotension, Hypoglycemia (central nervous system and glucocorticoid effects are more hindered)
Are Adrenal catecholamines essential for life?
NAUR for normal function just for stress situations
what is Pheochromocytoma
– tumor overproduces catecholamines
NO overt symptoms really ( headache • hypertension • sweating • palpitations • chest pain • anxiety • glucose intolerance • increased metabolic rate)