NMR Spectroscopy
1/41
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
42 Terms
Nuclear Magnetic Resonance
Nuclei possess charge and can spin
Spinning charge generates magnetic dipole
Magnetic Dipoles
characterized by nuclear magnetic spin quantum number “I”.
Three cases of “I”
1.I= 0
- Have an even number of protons and an even number of neutrons
- Do not spin - hence do not interact with applied magnetic field.
Examples: 12C, 16O
I = ½
Nuclei are NMR visible
examples: 1H, 13C (non-radioactive isotope of C, 1.06% natural abundance)
. I > 1/2
Examples: 2H and 14N – difficult to observe
diagram 1
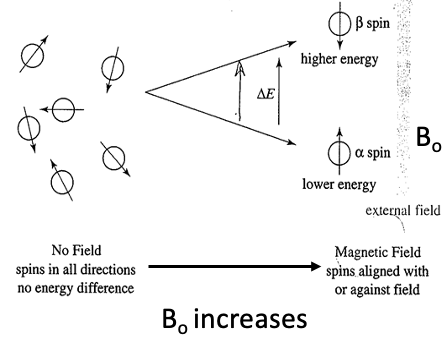
diagram 2
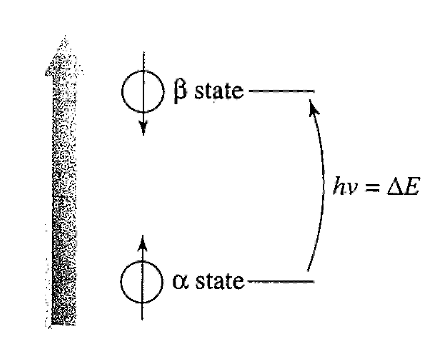
Planck’s equation:
∆E = hν
ν = γB_0/2π,
∆E = γ B_0h/2π
γ Boh/2π = hν
γ = gyromagnetic ratio; a constant that depends on the magnetic moment of the nucleus.
Bullet 1
•More nuclei aligned with externally applied magnetic field or lower energy α-spin state.
•∆E =
Energy difference between α and β-spin states
•Increasing the applied magnetic field =
Increasing ∆E between spin states
Nuclei can
absorb energy and flip from α-spin state to β-spin state.
When this happens, nucleus is said to be in RESONANCE with applied magnetic field, hence term “Nuclear Magnetic Resonance”.
The difference
in energy ΔE between two spin states corresponds to frequency in the Radiofrequency region of the EM spectrum.
downfield
left
these protons sense a larger effective magnetic field, so come into resonance at a higher frequency
upfield
these protons sense a smaller effective magnetic field, so come into resonance at a lower frequency
Example: CH3Cl Vs CH4
Electronegative Cl withdraws e-1 density from the ‘C’ and ‘H’ atoms – thus DESHIELDING them
Deshielding or Lesser shielding = more of the applied magnetic field strength experienced = higher (DOWNFIELD) Frequency absorbed
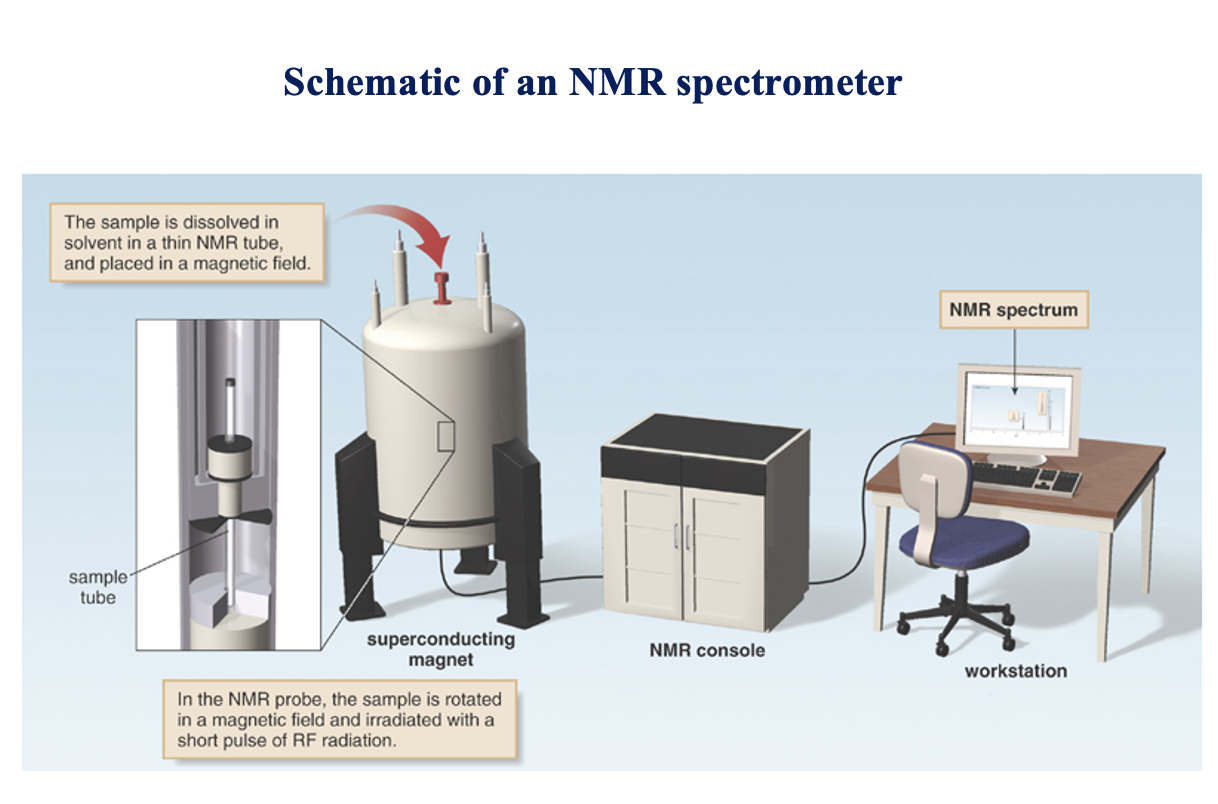
Schematic of an NMR spectrometer 1
The sample is dissolved in solvent in a thin NMR tube, and placed in a magnetic field.
Schematic of an NMR spectrometer 2
In the NMR probe, the sample is rotated in a magnetic field and irradiated with a short pulse of RF radiation.
NMR tech picture
Common NMR solvents:

A Typical NMR Sample Prep:
10-20 mg compound + 0.5-0.6 mL CDCl3
NMR spectrum:
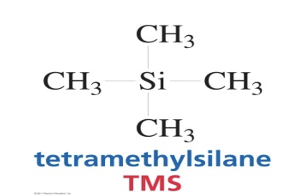
A plot of the intensity of an observed NMR signal Vs the frequency, measured relative to a reference compound; typically Tetramethylsilane (TMS)
TMS
Tert-butylmethylether
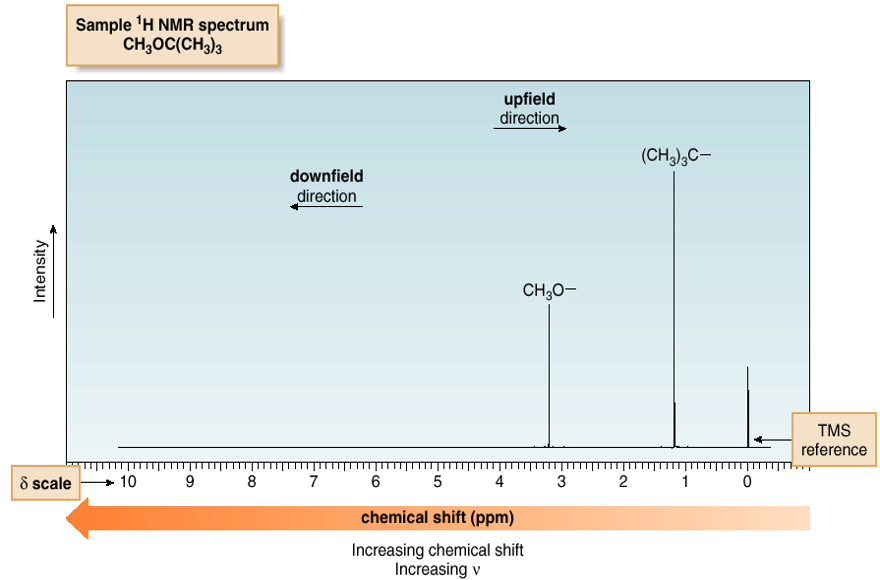

Advantages of TMS:
1. TMS HAS 12 equiv. H’s
2. NMR Signal due to protons in TMS appears as a singlet at 0 ppm (way upfield from other signals).
3. It is low-boiling and inert.
Chemical shift (Position of the Peak):
Frequency difference between the resonance observed for the nucleus and the resonance observed for the reference compound (usually TMS).
δ =

Degree of Unsaturation:
2 (No. of C atoms) + 2 + (No. of N atoms) – (No. of Halogen atoms) - (No. of H atoms)
2
For a DU ≥ 4 = 3 double bonds + 1 ring (= 1 Aromatic ring)
Chemically equivalent protons
protons in the same environment. Equivalent protons do not split each other’s signals.
Chemically in-equivalent protons
give more than one peak.
Area under an NMR signal is
proportional to the number of absorbing protons.
Height of each step
is proportional to the area under the peak which in turn is proportional to the number of absorbing protons.
Spin-spin splitting, occurs, peaks, # sigma bonds
•Spin-spin splitting occurs only between non-equivalent protons on the same carbon or adjacent carbons.
•A set of “n” non-equivalent protons splits the signal of a neighboring proton into (n+1) peaks.
•Splitting not generally observed for protons separated by more than 3 sigma bonds.
- Splitting by
“n” nuclei of spin quantum number “I” gives (2nI + 1) lines.
For protons where I= ½, this equation results in (n + 1) lines
Spacing (in Hz) between the lines in the multiplet = Coupling Constant (J) (units Hz)
Superscript before the symbol “J” represents the no. of inclusive bonds between the coupled nuclei
Coupled nuclei are identified by labels as subscripts after the symbol “J”
Ex. 2Jab = 2.4
Superscript before the symbol “J” represents
no. of inclusive bonds between the coupled nuclei
Coupled nuclei are identified by
labels as subscripts after the symbol “J”
Ex. 2Jab = 2.4
•OH protons
are not split and they do not split the NMR signal of adjacent protons signal appears as a singlet
Signal Multiplicities and Relative Intensities of Signal Peaks
Number of peaks Name
1 singlet (s)
2 doublet (d)
3 triplet (t)
4 quartet (q)
5 quintet
6 sextet
7 septet
>7 multiplet (m)
relative peak intensities: pascals triangle