113 [1] - Respiratory Complexes and ATP Synthesis
1/101
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
102 Terms
Mitochondria
major organ responsible for respiration
Mitochondria
“Powerhouse” of the cell
Hold specific machineries that is able to convert certain molecules to its ATP equivalent
Plays a major role in ATP synthesis
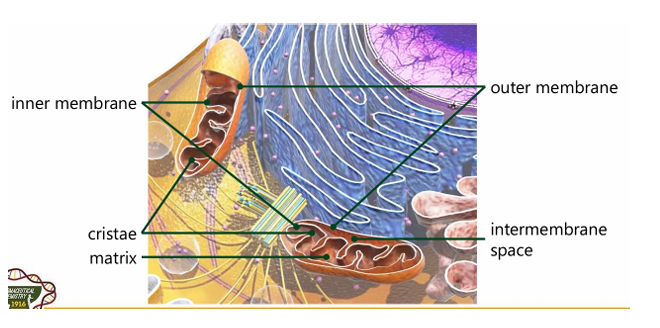
Outer membrane
Inner membrane
Intermembrane space
Matrix
Cristae
Different compartments of the mitochondria (5)
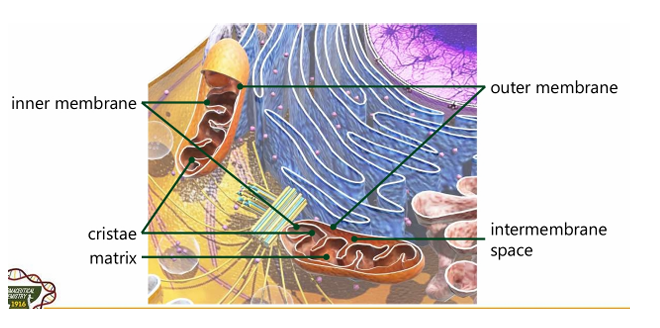
Innermembrane
[Compartment of Mitochondria]
Majority if not all of the enzymes of the Electron Transport chain reside here
Intermembrane space
[Compartment of Mitochondria]
Portion that divides inner and outer membrane. It is where protons in the Electron Transport Chain is accumulating; Reason why there is a proton gradient
Matrix
[Compartment of Mitochondria]
Hollow portion; Can be compared to “cytoplasm”; liquid portion
Cristae
[Compartment of Mitochondria]
Interfolding structure in the inner mitochondrial membrane
False.
Mitochondria is a double-membrane system (their own genetic material and ribosomes). Chloroplast is another membrane that has a double membrane for plant tissues. (Endosymbiotic Theory: Engulfing of bacteria, Pre-exist from a singular bacteria that is engulfed in another bacteria)
[T/F] Mitochondria is a single-membrane system.
True
[T/F] Mitochondrial genome are inherited exclusively from the mother, any genetic mutations in her mitochondrial DNA can directly impact the health and functioning of her offspring.
False.
Nuclear Genome Mutations: Mutations in the nuclear genome can be repaired effectively, and many are not detrimental due to the redundancy of genetic coding (e.g., multiple codons coding for the same amino acid). Moreover, if a mutation is harmful, it can lead to cell death, apoptosis, or diseases such as cancer, but the organism has mechanisms to cope with these changes.
Mitochondrial Genome Mutations: Mutations in the mitochondrial DNA are more problematic because they can lead to impaired energy production, affecting cell and tissue function. The consequences of such mutations can be more immediate and severe due to the essential role of mitochondria in cellular metabolism.
[T/F] Mutation in the mitochondrial genome is much “preferred” than in nuclear genome.
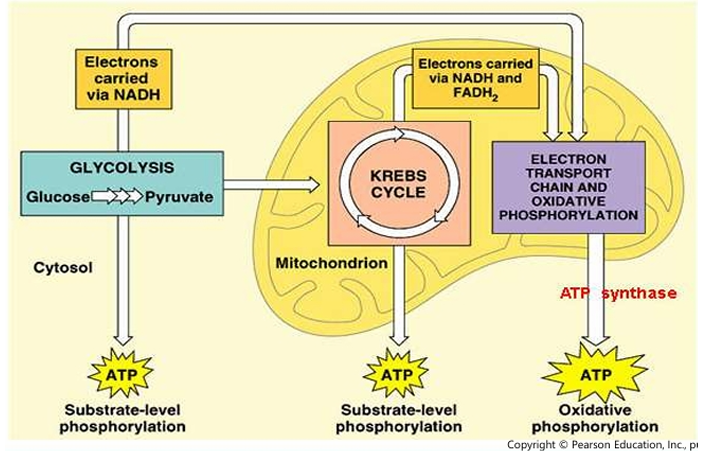
Overview of Cellular respiration
Overview of Cellular respiration
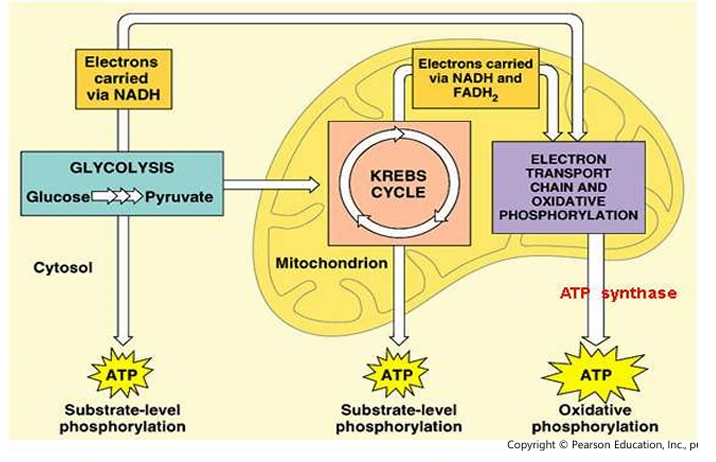
Substrate-level phosphorylation
Oxidative phosphorylation
Within the cell, there are 2 types of phosphorylation:
Substrate-level phosphorylation
[Type of Phosphorylation]
Transfer of phosphate group from 1 nucleoside triphosphate to another (e.g. GTP→ADP→ATP)
Oxidative phosphorylation
[Type of Phosphorylation]
Needs the electron transport chain to produce the ATP
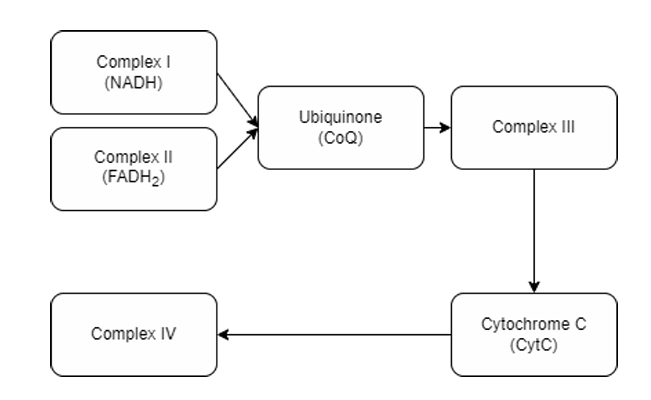
Electron transfer across the complexes (NADH, FADH₂, and complexes)
Four (Complex I, II, III, IV)
Note: 5 in a way because ATP Synthase is part of the ETC
How many complexes are there in Electron Transport Chain?
A. Complex I, II, III, IV (immobile complexes)
Integral membrane proteins in ETC
A. Complex I, II, III, IV (immobile complexes)
B. Ubiquinone
C. Cytochrome C
B. Ubiquinone
C. Cytochrome C
Mobile electron carriers in ETC
A. Complex I, II, III, IV (immobile complexes)
B. Ubiquinone
C. Cytochrome C
A. Complex I
NADH Dehydrogenase
A. Complex I
B. Complex II
C. Complex III
D. Complex IV
C. Complex III
Cyctochrome bc1
A. Complex I
B. Complex II
C. Complex III
D. Complex IV
B. Complex II
Succinate dehydrogenase
A. Complex I
B. Complex II
C. Complex III
D. Complex IV
D. Complex IV
Cytochrome c oxidase
A. Complex I
B. Complex II
C. Complex III
D. Complex IV
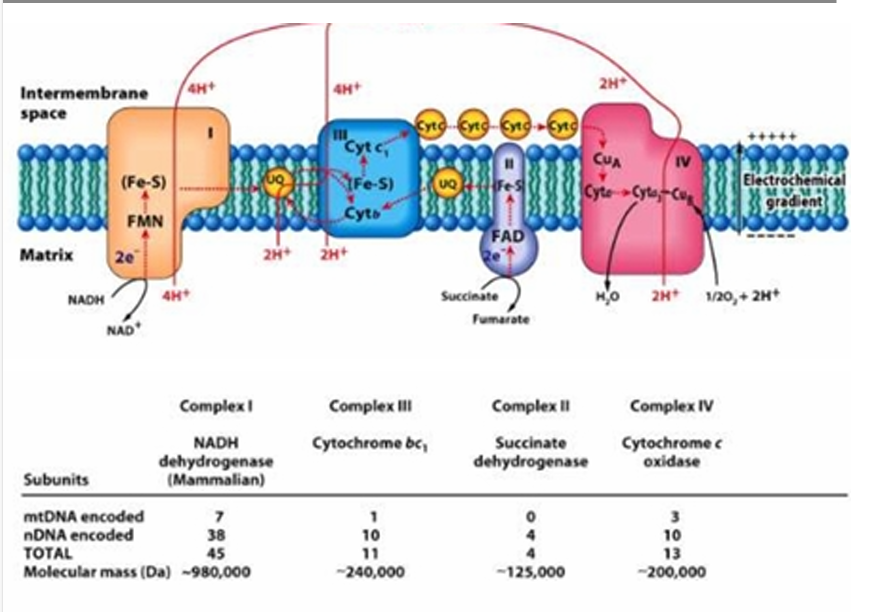
mtDNA = 7
nDNA = 38
TOTAL = 45
Give the number of mtDNA encoded and nDNA encoded in Complex I
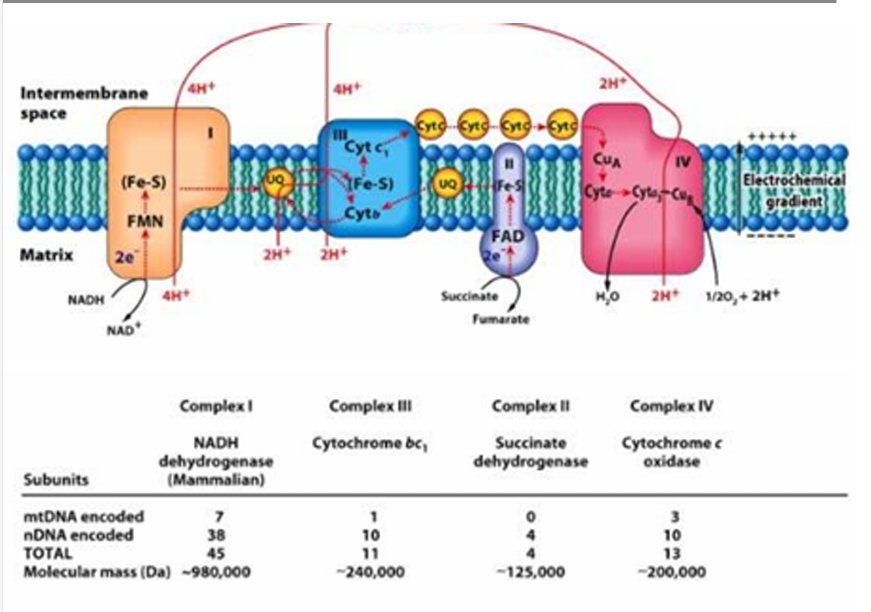
mtDNA = 0
nDNA =4
TOTAL = 4
Give the number of mtDNA encoded and nDNA encoded in Complex II
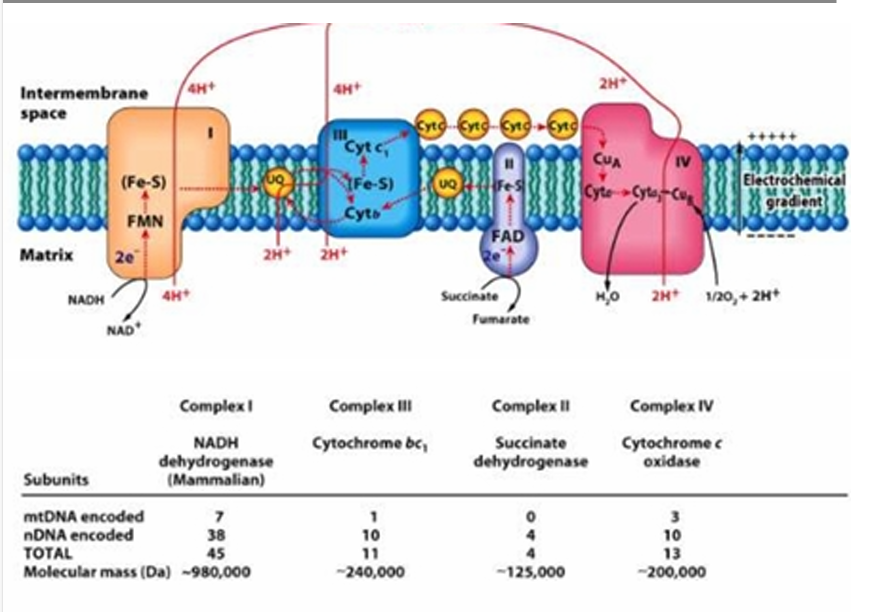
mtDNA = 1
nDNA = 10
TOTAL = 11
Give the number of mtDNA encoded and nDNA encoded in Complex III
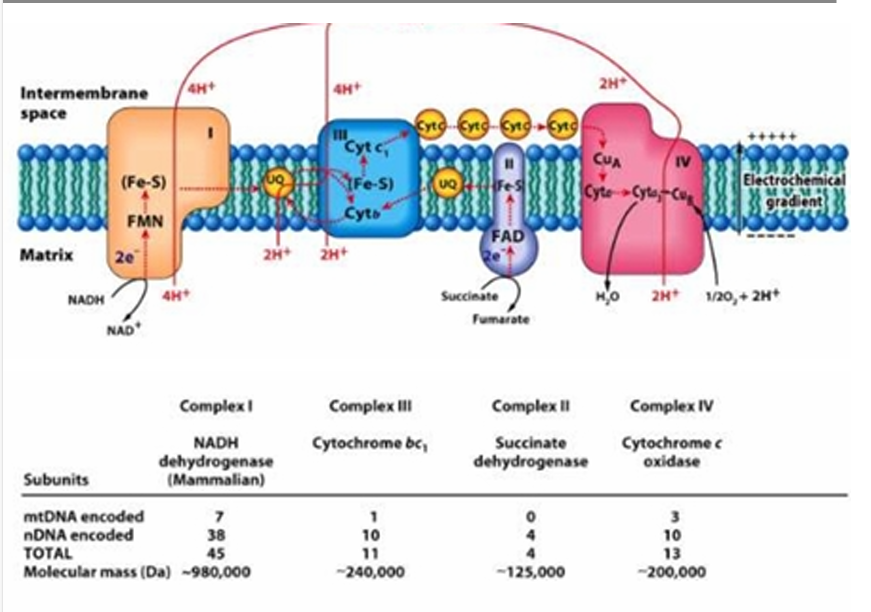
mtDNA =3
nDNA = 10
TOTAL = 13
Give the number of mtDNA encoded and nDNA encoded in Complex IV
Mitochondrial DNA
Nuclear DNA
Starting DNA for the transcript of the creation of these complex I, II, III, & IV (2)
True
[T/F] The majority of the DNA is found in the nuclear genome (from the nucleus)
True
[T/F] Proteins produced in the cytoplasm or even in the ribosomes (embedded in the endoplasmic reticulum) are essential for the proteins needed in the ETC process.
Electron carriers
They accept and give electrons during the ETC process
Flavoproteins
Cytochromes
Copper atoms
Ubiquinone (coenzyme Q)
Iron-sulfur proteins
Types of Electron Carriers (5)
Flavoproteins
[Type of Electron Carrier]
are polypeptides bound to either FAD or FMN
Flavin Adenine Dinucleotide
FAD
Flavin mononucleotide
FMN
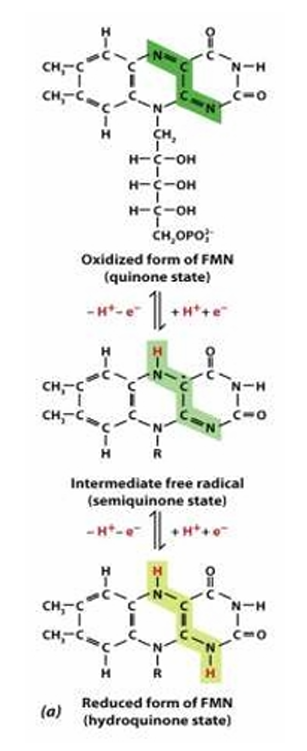
Structure:
Ribityl sugar/ backbone: reduced ribose
Isoalloxazine ring
Reactive site:
Isoalloxazine ring
Structure and Reactive site of Flavoproteins (FAD/FMN)
Cytrochromes
[Type of Electron Carrier]
Contain heme groups bearing Fe
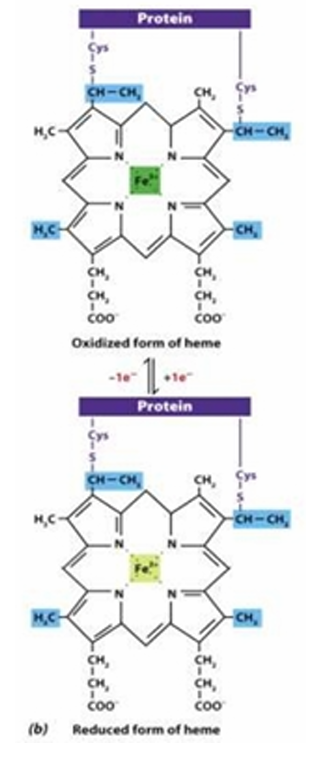
Structure: (e.g. of heme group)
Iron bound to porphyrin ring (also found in hemoglobin)
Reactive site: Iron (Fe2+ or Fe 3+)
Fe2+:reduced
Fe3+:oxidized
Structure and Reactive site of Cytochromes
Copper atoms
[Type of Electron Carrier]
Located within a single protein complex and alternate between Cu+/Cu2+ (e.g. cytochrome c oxidase, contain multiple copper ions often two or three)
E.g. Ceruloplasmin
Part of the oxidation process of chiral-containing groups
Ubiquinone (coenzyme Q)
[Type of Electron Carrier]
lipid-soluble molecule made of five-carbon isoprenoid units
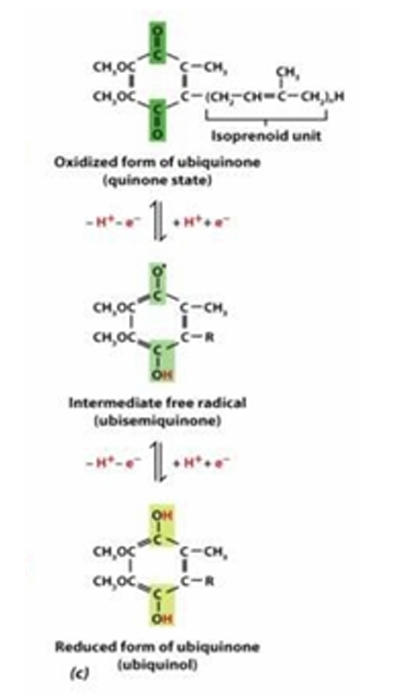
Reduced form: Ubiquinol
Ketone to Alcohol
Reactive site: Carbonyl carbon (partially positive or partially negative)
Reduced form and Reactive site of Ubiquinone (coenzyme Q)
Iron-sulfur proteins
[Type of Electron Carrier]
Contain Fe in association with inorganic sulfur
These carriers are arranged in order of increasingly positive redox potential.
Lattice describes how these clusters are incorporated into larger protein stabilizing their structures, overall conformation and function
Iron: part of the d metals
FMN → Coenzyme Q → Cytochrome C → Oxygen → Water
Arrangement of several carriers in the electron-transport chain: [FILL IN THE BLANKS]
Major molecule: [BLANK] → [BLANK] → [BLANK] → Oxygen (final electron acceptor) → [BLANK] (end process)
Choices: Water | Coenzyme Q | Oxygen | FMN | Cytochrome C
Nicotinamide adenine dinucleotide
NAD+
1,4- dihydro nicotinamide adenine dinucleotide
NADH
right, left
Carbon 3 (reactive site)
NAD+ & NADH
Reduced form: [left/right] :: Oxidized form : [left/right]
![<p>NAD+ & NADH</p><p>Reduced form:<span style="color: red"> <strong>[left/right]</strong></span><strong> </strong>:: Oxidized form : <span style="color: red"><strong>[left/right]</strong></span></p>](https://knowt-user-attachments.s3.amazonaws.com/59e7a2e8-c8d0-48ba-91f4-ed9824b2d921.png)
FAD and FADH2
Reactive site: isoalloxazine ring
What structure is shown in the picture?
Integral membrane protein
Part embedded in the membrane; Part protruded out in the membrane
Oxidoreductase enzyme
Enzyme in Complex I
Hydrophilic domain faces the aqueous environment (the matrix). It holds the binding site for Nicotinamide Adenine Dinucleotide + Hydrogen (NADH), where It accepts an electron from an NADH carrier.
NADH dehydrogenase oxidizes NADH → NAD+
The electron is then transferred to a Flavin Mononucleotide (FMN), forming NAD+and FMNH.
FMNH then transfers the electron to several iron-sulfur (FeS) clusters until it reaches the ubiquinone acceptor, which gets reduced by the electron, forming ubiquinol.
Mechanism of Complex I: NADH-COQ Oxidoreductase
intermembrane space = positive
matrix = negative
In normal orientation, intermembrane space holds the proton always [positively/negatively] charged. While Matrix are [postively/negatively] charged.
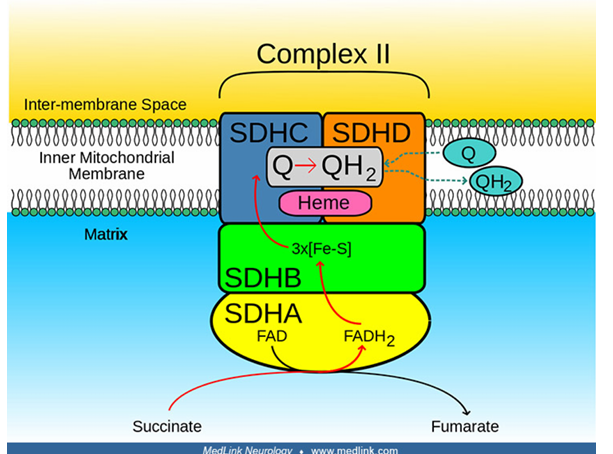
The electron donor is Succinate, which transfers the electron to Flavin Adenine Dinucleotide (FAD), forming fumarate and FADH2 .
The electron from FADH2 is then passed along in a series of iron-sulfur (FeS) clusters.
Succinate dehydrogenase itself does not contain heme
other proteins in the electron transport chain utilize heme for electron transport and anchorage
The endpoint is the Coenzyme Q (CoQ) or Ubiquinone, which is then reduced into Ubiquinol (QH2 ).
Mechanism of Complex II: Succinate-CoQ Oxidoreductase or Succinate Dehydrogenase
C. Both statements are true
I. Complex 1 electrons are independent from Complex 2 electrons
II. There are no pumping in the process of Complex 2
A. The first statement is true. The second statement is false.
B. The first statement is false. The second statement is true.
C. Both statements are true
D. Both statements are false
B. The first statement is false. The second statement is true.
I. Complex III is the only one of the 4 main complexes that does not pump electrons.
II. A pumping-type complex pumps out a proton to the intermembrane space once an electron is transferred.
A. The first statement is true. The second statement is false.
B. The first statement is false. The second statement is true.
C. Both statements are true
D. Both statements are false
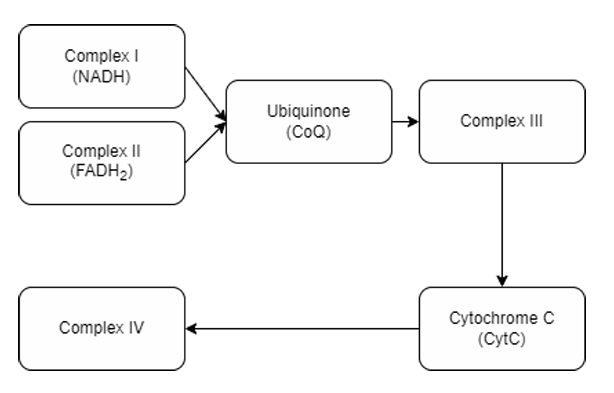
Complex III
Protein complex that accepts electrons from Complexes 1 and 2.
Electrons arrive in the form of Ubiquinol (QH2 ), which delivers two electrons (to an acceptor Q inside the complex) and protons and is converted back into Ubiquinone (CoQ). Ubiquinone then goes back to Complexes 1 and 2 to get more electrons.
The resident ubiquinone in the complex then transfers the electrons to iron clusters until it reaches the cytochrome-1 molecule.
The cytochrome-1 molecule will then transfer the electron to cytochrome c, which reduces it. It is then released into the intermembrane space.
Mechanism of Complex III: CoQ-Cyt C Oxidoreductase
C. Both statements are true
I. Electron transfer causes a change in the structure of the molecule.
II. To do another cycle, it needs to default back to its prior state.
A. The first statement is true. The second statement is false.
B. The first statement is false. The second statement is true.
C. Both statements are true
D. Both statements are false
Complex IV: Cyt C Oxidase
[ETC Complex]
Final electron acceptor to produce the final electron product
Reduced Cytochrome C transfers electrons to the Copper molecule
Copper will transfer the electrons to the Iron (can be Iron containing structure, or an Iron-Sulfur complex) molecule complexes, undergoing cascade pathways.
Cu>Fe>Fe>Cu
The Copper will transfer electrons to Oxygen (final acceptor) in the matrix, forming water.
Water is the final product
Mechanism of Complex IV: Cyt C Oxidase
False.
Oxygen is the oxidized form while Water is the reduced form
[T/F] Oxygen is the reduced form while Water is the oxidized form
ATP Synthesis
Major product of Complex IV
CO (competitive)
CN-, H2S (noncompetitive)
HCOOH, HN=N+=N- (uncompetitive)
HCOOH is formic acid
Inhibitors of Complex IV
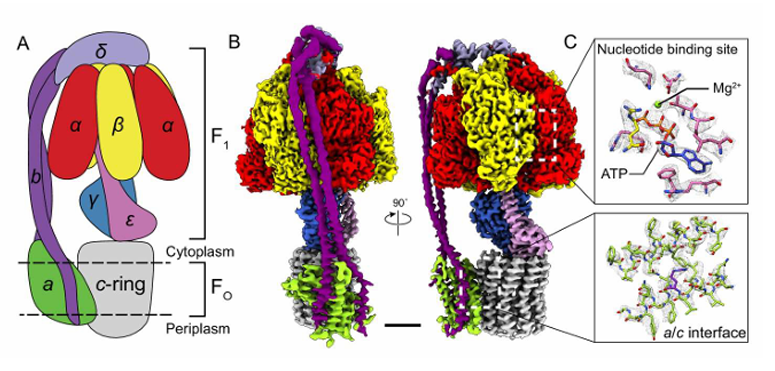
3 alpha (ɑ) subunit
3 beta (B) subunit
1 delta (δ) subunit
1 epsilon (ε) subunit
1gamma (𝛾)subunit
The action of ATP Synthase [5]
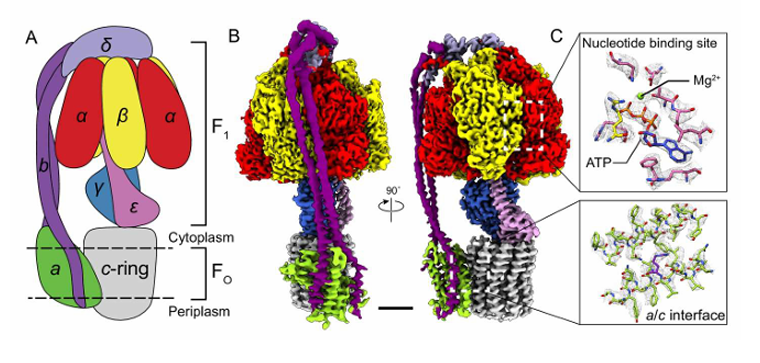
F1 - outside of the membrane, protruded out in the matrix
F0 - embedded in the membrane
Two major structures of ATP Synthesis
ATP Turbine or Proton Motive Force
Rotation of the F₀ component (often referred to as the C-ring) is driven by the flow of protons down their concentration gradient (from the intermembrane space into the matrix)
Open Conformation
Loose Conformation
Tight Conformation
O>L>T>T>O
Binding Change Mechanism of ATP Synthesis (3)
Open Conformation
[Binding Change Mechanism of ATP Synthesis]
NO ligands (ADP and Pi, in the matrix) are bound to the site. The site is open and ready to receive substrates.
Loose Conformation
[Binding Change Mechanism of ATP Synthesis]
The ligands bind to the site, leading to a conformational change, which allows the substrates to be held in place but not yet joined.
Tight Conformation
[Binding Change Mechanism of ATP Synthesis]
This conformation will allow ADP and Pi to join, forming ATP; The structure shifts back into an open conformation, releasing the newly formed ATP
True
[T/F] The flow of protons from the intermembrane space towards the mitochondrial matrix is what drives ATP production
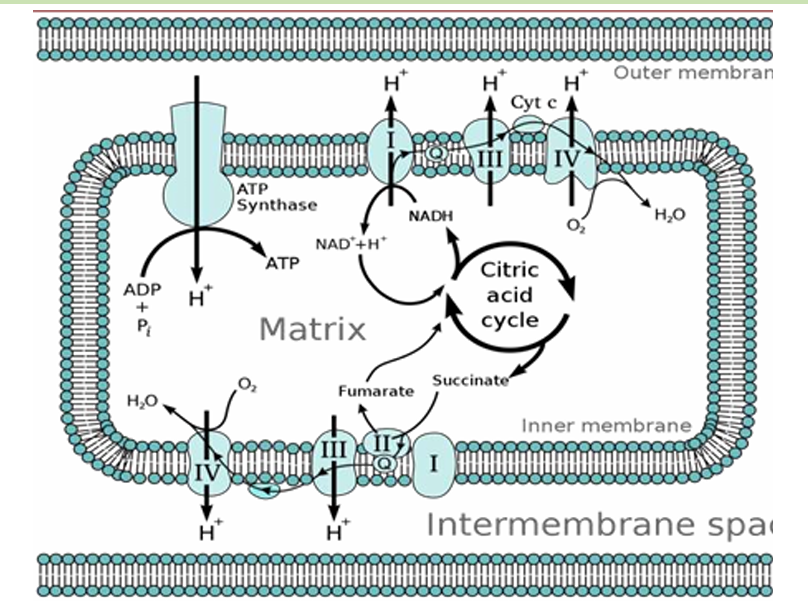
Chemiosmotic Coupling
How ATP is generated through the movement of electrons and protons across membranes.
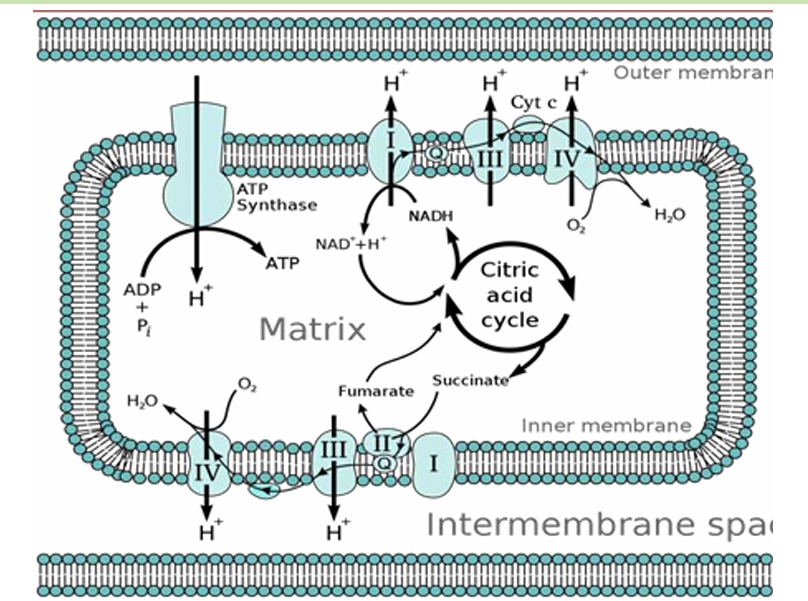
Chemiosmotic Coupling
The energy from a proton gradient is "coupled" to ATP synthesis
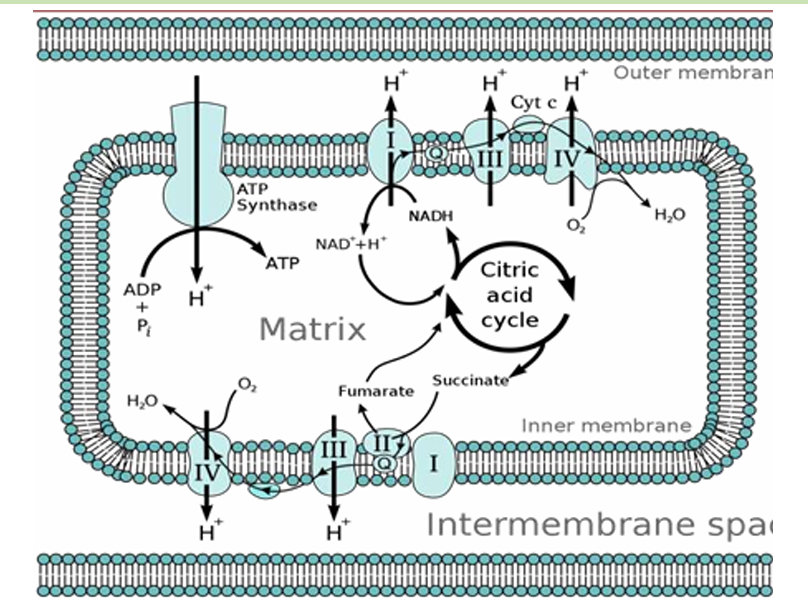
ADDTL INFOS
As electrons move through the ETC, they release energy, which is used to pump protons (H⁺ ions) from the mitochondrial matrix into the intermembrane space. This creates a proton gradient across the inner mitochondrial membrane—an essential setup for ATP production.
As protons accumulate in the intermembrane space, the concentration of protons becomes much higher there compared to the mitochondrial matrix. This creates two important gradients:
Chemical Gradient: More protons in the intermembrane space than in the matrix.
Electrical Gradient: The intermembrane space becomes positively charged relative to the matrix, creating a voltage difference across the membrane.
These two gradients together form what is called the proton motive force (PMF), which stores energy that can be used for ATP production.
The proton motive force drives protons back into the mitochondrial matrix through an enzyme complex called ATP synthase. This enzyme acts like a molecular turbine. As protons flow down their gradient through ATP synthase, the energy released from their movement is used to synthesize ATP from ADP and inorganic phosphate (Pi).
This process of producing ATP is called oxidative phosphorylation in cellular respiration and photophosphorylation in photosynthesis.
Chemiosmotic Coupling addt infos JUST READ***
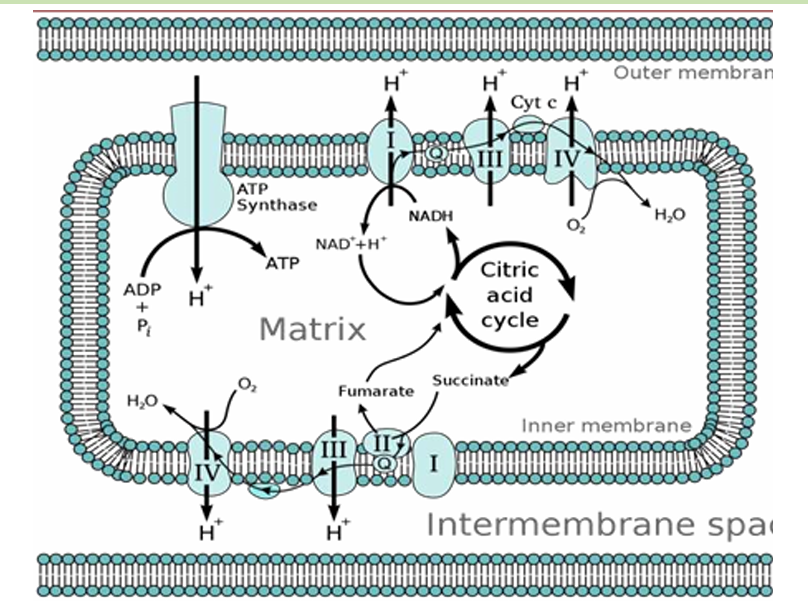
Processes like cellular respiration create a concentration gradient of protons—a high concentration in the Intermembrane Space and a low concentration in the Matrix.
Proton moves out
Protons move back in the matrix VIA F0 ATP Synthase
The movement of protons drives the ATP Production
Since there is a HIGH concentration of Protons in the Intermembrane Space and a LOW Concentration in the matrix; there is a movement back to the matrix (via F0 and F1) to achieve equilibrium.
Chemiosmotic Coupling Process
Mitochondrial uncoupling
a process where the proton gradient created across the mitochondrial inner membrane is disrupted, preventing ATP synthesis.
Mitochondrial uncoupling
protons bypass ATP synthase and re-enter the mitochondrial matrix through other pathways. This discharges the electrochemical gradient, which is required to power ATP synthesis. As a result, ATP production stops or decreases significantly.
Entry of Lipophilic Weak Acids followed by dissociation
Some uncoupling agents (like 2,4-dinitrophenol) are lipophilic weak acids. These molecules can cross the inner mitochondrial membrane in their protonated (neutral) form, enter the matrix, and then dissociate, releasing protons back into the matrix. This process neutralizes the proton gradient.
What protons enters the inner mitochondrial membrane that results to mitochondrial uncoupling?
Transport H+ across the inner membrane(to matrix)
Discharge electrochemical gradient that turns off ATP synthesis
Entry of lipophilic weak acids into the matrix followed by dissociation
Mechanism of Mitochondrial Uncoupling
No Proton Gradient, No ATP: Since ATP synthase relies on the proton gradient to produce ATP, its disruption means that little to no ATP is generated.
Energy Released as Heat: Instead of the energy being used for ATP production, it is dissipated as heat. This phenomenon is observed in certain physiological conditions (e.g., brown fat tissue in mammals for heat production) or when uncoupling agents are used (e.g., DNP was once used as a weight-loss drug because it increased metabolic rate and heat production, though it is dangerous and toxic).
Consequences of Uncoupling (2)
2,4-Dinitrophenol (DNP): A protonophore that can transport protons across the mitochondrial membrane, disrupting ATP synthesis and releasing energy as heat.
Carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (FCCP): Another potent uncoupling agent that collapses the proton gradient by transporting protons into the matrix, thereby preventing ATP production.
Protonophores, which accept protons (sequesters protons), like 2,4-Dinitrophenol, is capable of entering the membrane. This causes the protons to bypass ATP synthase and flow back across the membrane without driving ATP production–decreasing ATP production (resulting in energy being released as heat instead of being stored in ATP).
Examples of Uncoupling agents (2)
Brown fat
contains a much higher concentration of mitochondria compared to white fat. Mitochondria are responsible for energy production, and the large number of these organelles gives brown fat its brown color due to the presence of cytochromes, proteins involved in the electron transport chain.
More Triacylglycerol (TAG)
Brown fat cells contain smaller lipid droplets compared to white fat, but the higher mitochondrial content allows for more efficient mobilization of stored fats, which are burned to generate heat.
Heat generation via non-shivering thermogenesis (“biological heating pad”)
Regulated uncoupling of oxidative phosphorylation with the help of
Thermogenin
an uncoupling protein found exclusively in brown fat
Inhibitors: ATP, ADP, GTP, GDP
White Fat:
Energy Storage: White fat cells are mainly responsible for storing energy in the form of large lipid droplets (triacylglycerol). Unlike brown fat, white fat has fewer mitochondria and does not specialize in heat production.
Brown fat vs White fat
False.
Brown fat : heat generation :: White fat : energy storage
[T/F] Brown fat : energy storage :: White fat : heat generation
Cytochromes
The brown color of brown fat is due to the presence of
Brown fat
White fat
Two types of fat in the body
a) citrate synthase
b) aconitase
c) isocitrate dehydrogenase (IRREV)
d) α-ketoglutarate dehydrogenase (IRREV)
e) succinate thiokinase
f) succinate dehydrogenase
g) fumarase
h) malate dehydrogenase
Enzymes involved in Citric Acid Cycle (8)
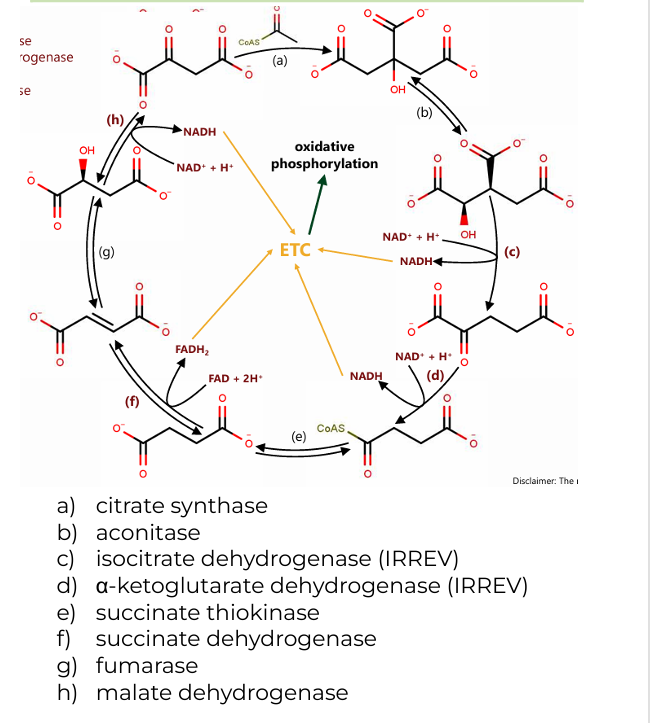
2 H2O(citrate synthase, fumarase)
(a): One water molecule is added when acetyl-CoA combines with oxaloacetate to form citrate.
(g): Another water molecule is consumed during the conversion of fumarate to malate
CoASH (a-ketoglutarate dehydrogenase)
CoASH is released during the conversion of α-ketoglutarate to succinyl-CoA and is also involved in forming acetyl-CoA before the cycle begins.
GDP+Pi (succinate thiokinase)
In the conversion of succinyl-CoA to succinate, GDP and Pi (inorganic phosphate) are used to generate GTP (which is energetically equivalent to ATP).
Co-substrates in CAC (2)
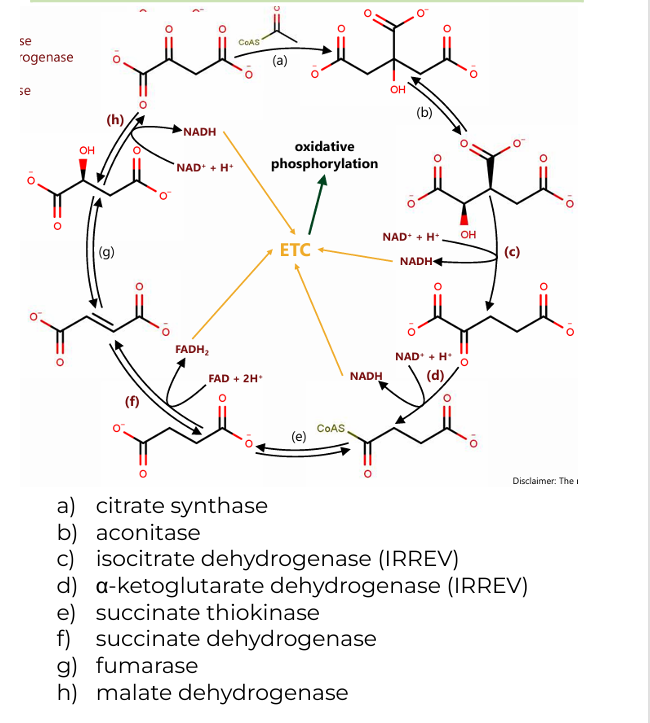
2 CO2 (isocitrate dehydrogenase, α-ketoglutarate dehydrogenase)
One during the conversion of isocitrate to α-ketoglutarate.
Another during the conversion of α-ketoglutarate to succinyl-CoA.
2 CoASH (a-ketoglutarate dehydrogenase)
One during the formation of succinyl-CoA from α-ketoglutarate.
The other when acetyl-CoA enters the cycle and combines with oxaloacetate.
GTP (succinate thiokinase)
One molecule of GTP is produced during the conversion of succinyl-CoA to succinate, which can be converted to ATP.
Byproducts of CAC
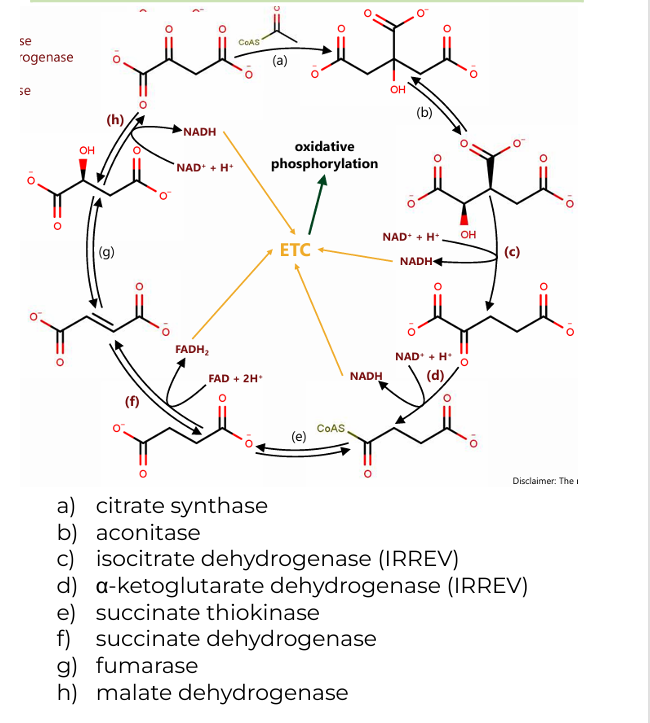
3 NADH (c, d, h)
(c): Isocitrate → α-ketoglutarate (produces NADH and CO₂)
(d): α-ketoglutarate → succinyl-CoA (produces NADH, CO₂, and CoASH)
(h): Malate → oxaloacetate (produces NADH)
1 FADH2(f)
(f): Succinate → Fumarate (produces FADH₂)
1 round of Acetyl CoA, how many NADH and FADH2?
2.5 ATP
How many ATP in NADH
1.5
How many ATP in FADH2
For each acetyl-CoA that enters the citric acid cycle, the yield is:
3 NADH (which can generate ~7.5 ATP in the electron transport chain)
1 FADH₂ (which can generate ~1.5 ATP)
1 GTP (which is equivalent to 1 ATP)
Byproducts: 2 CO₂ molecules and CoASH.
This totals ~10 ATP produced per round of the citric acid cycle. The cycle repeats for each molecule of acetyl-CoA, meaning one glucose molecule (which yields two acetyl-CoA molecules) leads to approximately 20 ATP from the citric acid cycle alone.
Total ATP produced per round of CAC
The cycle repeats for each molecule of acetyl-CoA, meaning one glucose molecule (which yields two acetyl-CoA molecules) leads to approximately 20 ATP from the citric acid cycle alone.
Total ATP produced per CAC
D. NADH and FADH₂ are high, while the levels of NAD⁺ and FAD are low.
When the cell is at rest or low in energy demand (Initial state, catabolism off), the levels of
A. NADH and FADH₂ are low, while the levels of NAD⁺ and FAD are low.
B. NADH and FADH₂ are low, while the levels of NAD⁺ and FAD are high.
C. NADH and FADH₂ are high, while the levels of NAD⁺ and FAD are high.
D. NADH and FADH₂ are high, while the levels of NAD⁺ and FAD are low.
B. NADH and FADH₂ are low, while the levels of NAD⁺ and FAD are high.
When the cell requires energy (e.g., during exercise), this system "turns on" catabolic pathways.
A. NADH and FADH₂ are low, while the levels of NAD⁺ and FAD are low.
B. NADH and FADH₂ are low, while the levels of NAD⁺ and FAD are high.
C. NADH and FADH₂ are high, while the levels of NAD⁺ and FAD are high.
D. NADH and FADH₂ are high, while the levels of NAD⁺ and FAD are low.
1. Functional Differences of Complexes I and II and Their Connections to Other Complexes
Key Differences:
Electron Donors: Complex I receives electrons from NADH, while Complex II receives electrons from FADH₂ (produced from succinate oxidation in the citric acid cycle).
Proton Pumping: Complex I contributes to the proton gradient by pumping protons, while Complex II does not.
Both Complexes I and II feed electrons into ubiquinone (Coenzyme Q), which connects them to Complex III in the electron transport chain.
2. Binding-Change Mechanism of ATP Synthase
The binding-change mechanism explains how ATP synthase synthesizes ATP from ADP and inorganic phosphate (Pi) as protons flow through the enzyme. ATP synthase has three active sites that cycle through three different conformations:
Loose (L) State: In this state, the site binds ADP and Pi loosely, preparing them for the next step.
Tight (T) State: The site undergoes a conformational change that brings ADP and Pi close enough together to form ATP. This is where ATP is synthesized.
Open (O) State: In this state, ATP is released from the site, and the cycle resets.
As protons (H⁺) flow through the F₀ subunit of ATP synthase, the rotational movement generated causes conformational changes in the F₁ subunit, cycling each active site between these three states. This process allows ATP to be produced in the T-state and released in the O-state.
3. Mitochondrial Chemiosmotic Coupling and the Role of ThermogeninChemiosmotic Coupling:
This concept, proposed by Peter Mitchell, describes how the electron transport chain (ETC) and ATP synthesis are coupled via a proton gradient. As electrons flow through the ETC, protons are pumped across the inner mitochondrial membrane, creating a proton motive force. This force consists of:
Chemical gradient: Difference in proton concentration (pH gradient).
Electrical gradient: Difference in charge across the membrane.
The proton motive force drives ATP synthesis as protons flow back into the mitochondrial matrix through ATP synthase, providing the energy needed to convert ADP and Pi into ATP.
Thermogenin (Uncoupling Protein 1, UCP1):
Thermogenin is a protein found in brown adipose tissue that uncouples oxidative phosphorylation from ATP synthesis by allowing protons to flow back into the matrix without passing through ATP synthase.
Effect on Chemiosmotic Coupling: Thermogenin dissipates the proton gradient by allowing protons to "leak" across the inner mitochondrial membrane. Instead of producing ATP, the energy from the proton gradient is released as heat. This process is known as non-shivering thermogenesis, which helps maintain body temperature in cold environments.
Thus, thermogenin disrupts the normal chemiosmotic coupling by uncoupling proton flow from ATP synthesis, converting the energy into heat instead of ATP.
Guide Questions: BASAHIN MO NLNG NAKAKATAMAD NA 😉
1) What are the functional differences of Complexes I and II? How are they connected to the other complexes?
2) How does the binding-change mechanism of ATP synthase work?
3) What is mitochondrial chemiosmotic coupling? How does thermogenin affect such a phenomenon?
False.
Both aerobic respiration and anaerobic respiration use an electron transport chain. The difference is that aerobic respiration uses oxygen as the final electron acceptor, while anaerobic respiration uses other molecules such as nitrate, sulfate, or carbon dioxide.
[T/F] Only aerobic respiration uses electron transport chain
B. 3 molecules of ATP
How many molecules of ATP are produced after one rotation of ATP Synthase Complex?
A. 1 molecules of ATP
B. 3 molecules of ATP
C. 5 molecules of ATP
D. 10 molecules of ATP
B. Potential Energy
This potential energy is stored in the form of a proton gradient (proton motive force) across the inner mitochondrial membrane (in eukaryotes) or the plasma membrane (in prokaryotes). As protons flow back through ATP synthase from an area of high concentration to an area of low concentration, this potential energy is converted into mechanical energy, which drives the synthesis of ATP from ADP and inorganic phosphate (Pi).
The energy used to drive ATP synthase
A. Chemical Energy
B. Potential Energy
C. 4 electrons
A total of 4 electrons are used to reduce one molecule of oxygen (O₂) to form two molecules of water (H₂O) in the electron transport chain.
How many electrons are used to form water in the reduction of oxygen in the electron transport chain?
A. 1 electron
B. 2 electrons
C. 4 electrons
D. 10 electrons
A. NADH levels will increase
When an inhibitor is present at Complex IV (cytochrome c oxidase) in the electron transport chain, the transfer of electrons to oxygen is blocked. As a result, the entire electron transport chain becomes backed up because electrons cannot be passed from Complex III to Complex IV.
Due to this blockage, NADH levels will increase because NADH cannot be oxidized back to NAD⁺ at Complex I, as the electron flow is halted. This accumulation of NADH ultimately slows down or stops the citric acid cycle, as it depends on the availability of NAD⁺ to continue operating efficiently.
In complex IV, when an inhibitor is present, what happens to the NADH levels?
A. NADH levels will increase
B. NADH levels will decrease
C. No change in NADH levels
True
[T/F] The proton gradient (also known as the proton motive force) across the inner mitochondrial membrane solely drives ATP synthase.
False.
The flow of electrons through the electron transport chain (ETC) is responsible for creating the proton gradient by pumping protons (H⁺ ions) across the inner mitochondrial membrane. However, it is the proton gradient (proton motive force) that directly drives ATP synthase, not the electron flow itself.
[T/F] The flow of electrons directly drives ATP synthase.