Lecture 13 Antibody Structure and Function Part 1
1/65
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
66 Terms
Antibodies (immunoglobulins, Ig)
Belong to the globulin protein class
-Can be membrane-bound or secreted
Membrane-bound = Part of the BCR on the surface of B-cells
Secreted = Free-floating antibodies produced by plasma cells in bone marrow
-Several key Ig properties/features are critical for immune response: specificity and biological effects
Specificity
Attributed to hypervariable complementarity- determining regions
-Only reacts with substances containing a particular antigenic structure
-Prompted evolution of diverse antibody system
Biologic effects
-Toxin neutralization
-Microorganism immobilization
-Viral neutralization
-Agglutination
-Precipitate formation
-Complement-mediated opsonization
What enzyme is used to split immunoglobulin molecules?
Papain
What are the two fragments produced by papain that retain the ability to bind to antigens?
Fab (Fragment antigen-binding) fragments
What is the third fragment produced by papain that can be crystallized but cannot bind to antigens?
Fc fragment (fragment crystallizable)
-responsible for antibody biological functions
What is the role of mercaptoethanol in breaking immunoglobulin disulfide bonds?
Used to break immunoglobulin disulfide bonds
What are the components of an immunoglobulin molecule?
Consists of two small, identical light chains and two large, identical heavy chains
How are the heavy and light chains held together in an immunoglobulin molecule?
Held together by several disulfide bonds
What creates the immunoglobulin-fold domains in heavy and light chains?
Intrachain disulfide bonds create immunoglobulin-fold domains
Major light chain classes
κ-> Chromosome 2 (60-67% of light chains)
λ-> Chromosome 22 (33-40% of light chains)
-In any immunoglobulin molecule, the light chains are always either both κ or both λ, but never one of each (κ2 or λ2)
How are major heavy chain classes distinguished from each other?
Based on constant regions, differing in protein sequence, carbohydrate content, and size
What are the genes encoding constant regions for heavy chains called?
Cγ, Cμ, Cα, Cδ, and Cε, giving rise to the 5 isotypes
Are the heavy chains in any antibody molecule identical?
Yes, they are always identical (γ2, μ2, α2, δ2, ε2)
Intrachain disulfide bonds
form regular, repeated loops of amino acids along the heavy and light chains
-each loop forms a compactly folded globular domain
Domains
Each is designated by a letter (where it is on a light or heavy chain) and a number (indicates position)
-First domain (light and heavy): Variable region (VH or VL)
-Light chains have two domains each
-Heavy chains have four or five domains separated by a short, unfolded section at the hinge region
What is the composition of the hinge region in an antibody?
Composed of a short segment of amino acids (cysteine and proline residues) between the CH1 and CH2 heavy chain regions
What is the role of cysteines in the hinge region of an antibody?
Cysteines (hydrophilic) are involved in interchain disulfide bonding
What is the role of prolines in the hinge region of an antibody?
Prolines (hydrophobic) prevent globular structure folding
Why is flexibility important in the hinge region of an antibody?
Provides flexibility between the two Fab arms of the antibody - Allows for the accommodation of two identical antigenic epitopes separated by a fixed distance
What is the Variable Region of an antibody?
Section of the antibody molecule that specifically binds to the epitope of an antigen
Where does the greatest variability in the Variable Region occur?
In three regions of the light and heavy chains, called hypervariable regions
What are the complementarity-determining regions (CDRs) in the Variable Region responsible for?
Contacts amino acids in the center of the peptide, with CDR1, CDR2, and CDR3 playing key roles
Framework regions
less-variable sections of the antibody between the hypervariable regions
Hypervariable regions arranement
arranged in the 3-D antibody molecule to form the antibody-binding site (CDR)
-CDR variability provides the shape diversity required for antibodies to recognize epitopes of different specificities (Antigen-antibody forces are weak, non-covalent interactions)
Binding affinities
Differences in the number and types of binding forces available to bind identical antigens to the different binding sites of two antibodies
Redundancy
A particular antibody-binding site with the ability to combine with two (or more) diverse epitopes
Immunoglobulin variants
isotype, allotype, idiotypes
Isotype
Differences between constant regions due to the usage of different heavy and light chain genes.
How do effector mechanisms activate in antibodies?
When a specific antibody molecule combines with a specific antigen.
What determines the effector mechanisms in antibodies?
The class of immunoglobulin (isotype) found on the heavy chain.
How do different antibodies with the same antigenic specificity trigger different responses?
The constant regions of the heavy chain trigger different biological responses.
Allotype
Differences due to different alleles on the same constant region gene
How do allotypic differences manifest in the population?
Heavy and Light chain alleles for a specific constant region may be present in some members of the population and absent in others.
What changes do allotypic differences at known gene loci usually result in?
Changes to one or two amino acids (highly conserved).
How do allotypic differences typically affect immunoglobulins' ability to bind to antigens?
Rarely affects immunoglobulins from binding to antigen.
Known allotypic markers
Gm= Group on the γ chain of IgG
Km= Group on the κ chain of light chains
Am= Group on the α chain of IgA
What are idiotypes?
Differences due to specific rearranged VH and VL genes within a given isotype.
How is the configuration of amino acids in the antigen-binding site related to idiotypes?
The configuration is specific and not present in other antibody molecules.
What is the significance of the antigen-binding site in idiotypes?
It should be immunogenic and capable of stimulating an immunologic response against itself in another individual.
What are anti-idiotype responses?
Responses primarily to each other, occurring normally in individuals, and resulting from idiotypes.
Public or cross-reacting idiotypes
-Anti-idiotypic antibodies react with several different antibodies for the same epitope
-Private idiotypes= Anti-idiotypic antibodies react to one epitope antibody
What is IgG?
Predominant immunoglobulin in blood, lymph fluid, cerebrospinal fluid, and peritoneal fluid
What are the subclasses of IgG?
IgG1, IgG2, IgG3, and IgG4 (named for order of abundance)
What percentage of total protein in adult human serum does IgG represent?
About 15%
How is IgG distributed in the body?
Equally between intravascular and extravascular spaces
What is the half-life of IgG1, IgG2, and IgG4?
Approximately 23 days (longest Ig half-life)
-IgG3 has half-life of 7 days
For what purpose is IgG most suitable?
Passive immunization by antibody transfer
What happens to IgG half-life as its concentration in the serum increases?
IgG half-life decreases to 15-20 days or less
What is the role of FcRp (Brambell receptor) in IgG biology?
FcRp is found in cellular endosomes and selectively recycles endocytosed IgG back to circulation
How does the rate of IgG catabolism change with increasing IgG concentration in the serum?
The rate of IgG catabolism increases
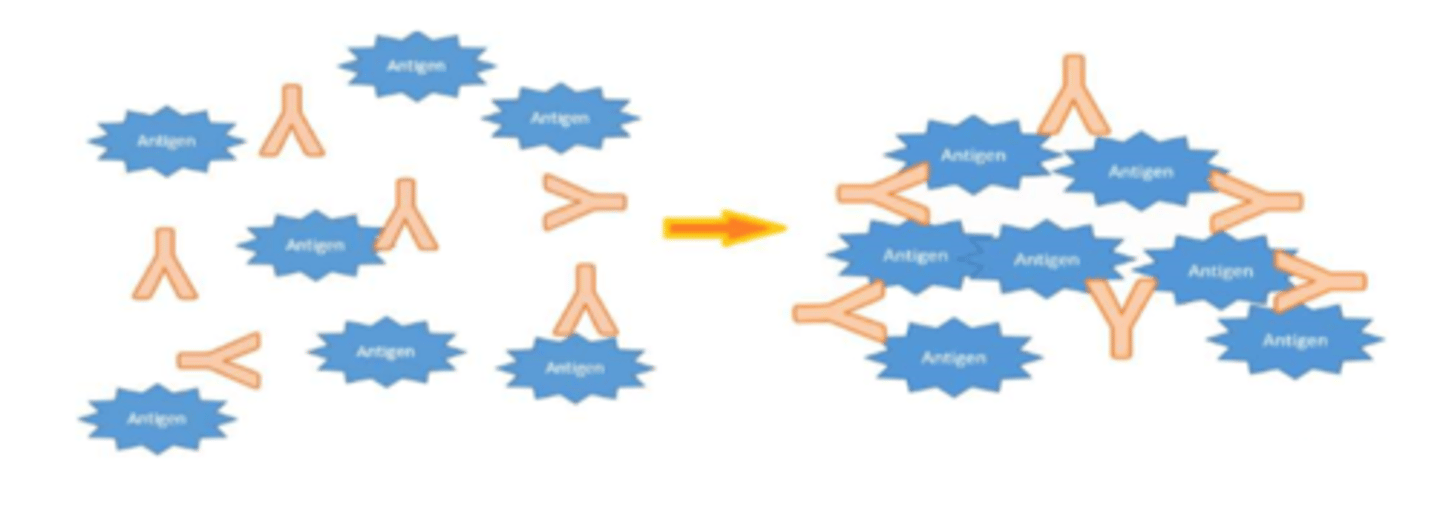
Agglutination
the clumping of particulate (insoluble) antigens (ex. microorganisms)
-Insoluble antigen-antibody complexes are easily phagocytized and destroyed
-IgG molecules aggregate and can combine with antigen.
Which immunoglobulin can pass through the placenta?
IgG
-except IgG2
What is the role of IgG in pregnancy?
Enables mother to transfer her immunity to the fetus through the placenta.
What is the IgG protection receptor expressed on placental cells?
FcRn
At what stage of pregnancy does the fetus begin to synthesize IgM and trace IgA?
After the fifth month of pregnancy
How is fetal resistance to infection mainly conferred?
By the mother's IgG
What is the main biological property of IgG?
IgG is an opsonizing antibody.
opsonizing= a process that helps the immune system identify and destroy pathogens and old cells. It involves coating pathogens with opsonins, which are proteins or lipids produced by the body, to help phagocytes find, attach to, and break down the pathogens.
How does the Fc portion of IgG contribute to its opsonizing property?
The Fc portion of the IgG molecule confers the opsonizing property.

When do IgG antibodies bind to specific antigenic epitopes?
IgG antibodies will bind to specific antigenic epitopes via Fab portions within a week-10 days of the antibody response.
What do phagocytic cells have that interact with IgG antibodies?
Phagocytic cells bear receptors for the Fc portion of the IgG molecule.
How do phagocytes adhere to antibody-coated bacteria?
Phagocytes adhere to the antibody-coated bacteria through the Fc receptors.
What is the net effect of phagocytes interacting with IgG-coated bacteria?
The net effect is a zipper-like closure of the phagocytic cell surface membrane.
Antibody-dependent Cell-mediated Cytotoxicity
-IgG plays a critical role
-IgG Fab binds to the target cell
-Fc binds with specific Fc receptors on natural killer (NK) cells
IgG focuses NK cells on the target
-NK cells DO NOT phagocytize cell
-Kill cells with:
-Granzymes (cytoplasmic granules)
-Respiratory burst (antimicrobial peptides (AMPs) or toxic substances)
IgG Biological Properties
-Complement activation - IgG plays a role in classical complement pathway
-Toxin Neutralization- IgG neutralizes toxins; Isotype of choice for passive immunization
-Bacterial Immobilization- IgG clumps around flagella of bacteria to prevent movement
-Virus Neutralization- IgG inhibits viral attachment to decrease chance of infection