biology quiz 3
1/113
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
114 Terms
kinetic energy
The energy of motion
potential energy
The energy matter possesses bc of its location or structure
oxidation
chemical reaction in which electrons are lost
think leo: loss of electron oxidation
reduction
chemical reaction in which electrons are added
think ger - gain electron reduction
anabolism
Metabolic pathways that consume energy to build complicated molecules from simpler ones (ex: synthesis of amino acids from simpler molecules)
catabolism
Metabolic pathways that release energy by breaking down complex molecules to simpler compounds (ex: cellular respiration: breaks down glucose and other organic fuels in presence of oxygen to carbon dioxide and water)
Explain the First Law of Thermodynamics and its relationship to biological systems.
The First Law of Thermodynamics states that the energy of the universe is constant. In other terns, it states that energy can be transferred and transformed but not created or destroyed. An example of how this relates to biological systems is that plants convert sunlight to chemical energy but do not produce or destroy it.
Explain the Second Law of Thermodynamics and its relationship to biological systems
The Second Law of Thermodynamics states that every energy transfer or transformation increases the entropy of the universe. In simpler terms, it says that as energy is transferred or transformed, wasted energy (such as energy lost as heat) is increased. An example of how it relates to biological systems is that consumers only get a small percent of the energy stored in their food because energy is released as heat between trophic levels.
what is meant by change in free energy?
free energy is energy that can do work when temperature and pressure are uniform, as in a living cell; measure of a systems instability, its tendency to change to a more stable state
free energy change of a reaction tells us whether or not the reaction occurs spontaneously
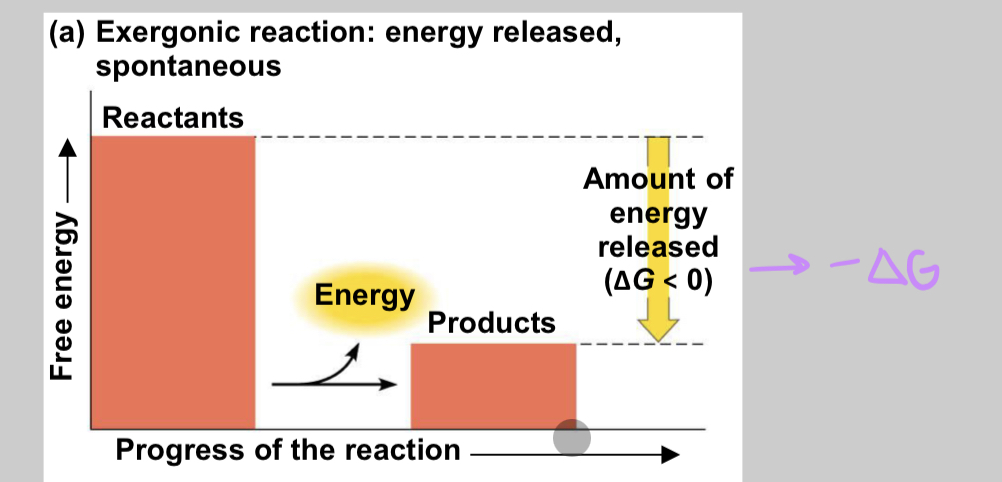
exergonic reactions
energy is released into surroundings, spontaneous
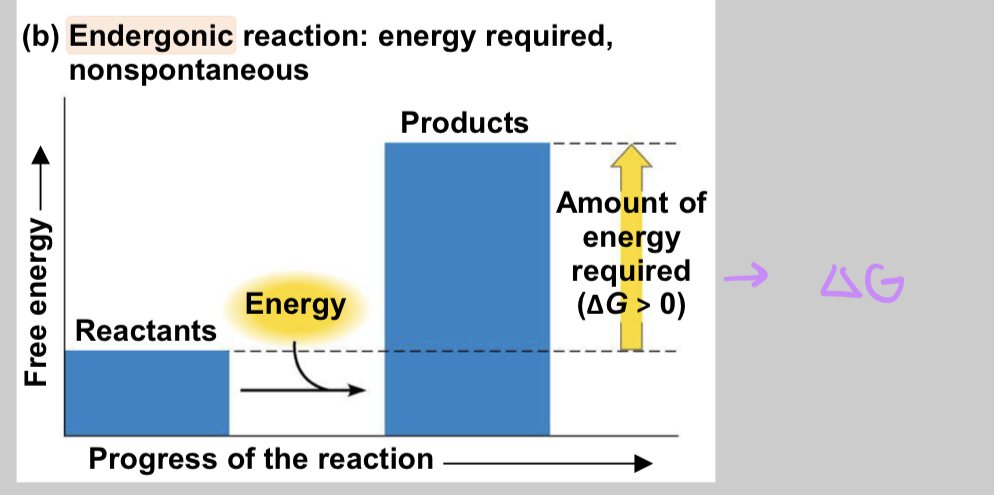
endergonic reactions
energy is absorbed from surroundings
what is a catalyst?
chemical agent that speeds up a reaction without being consumed by the reaction
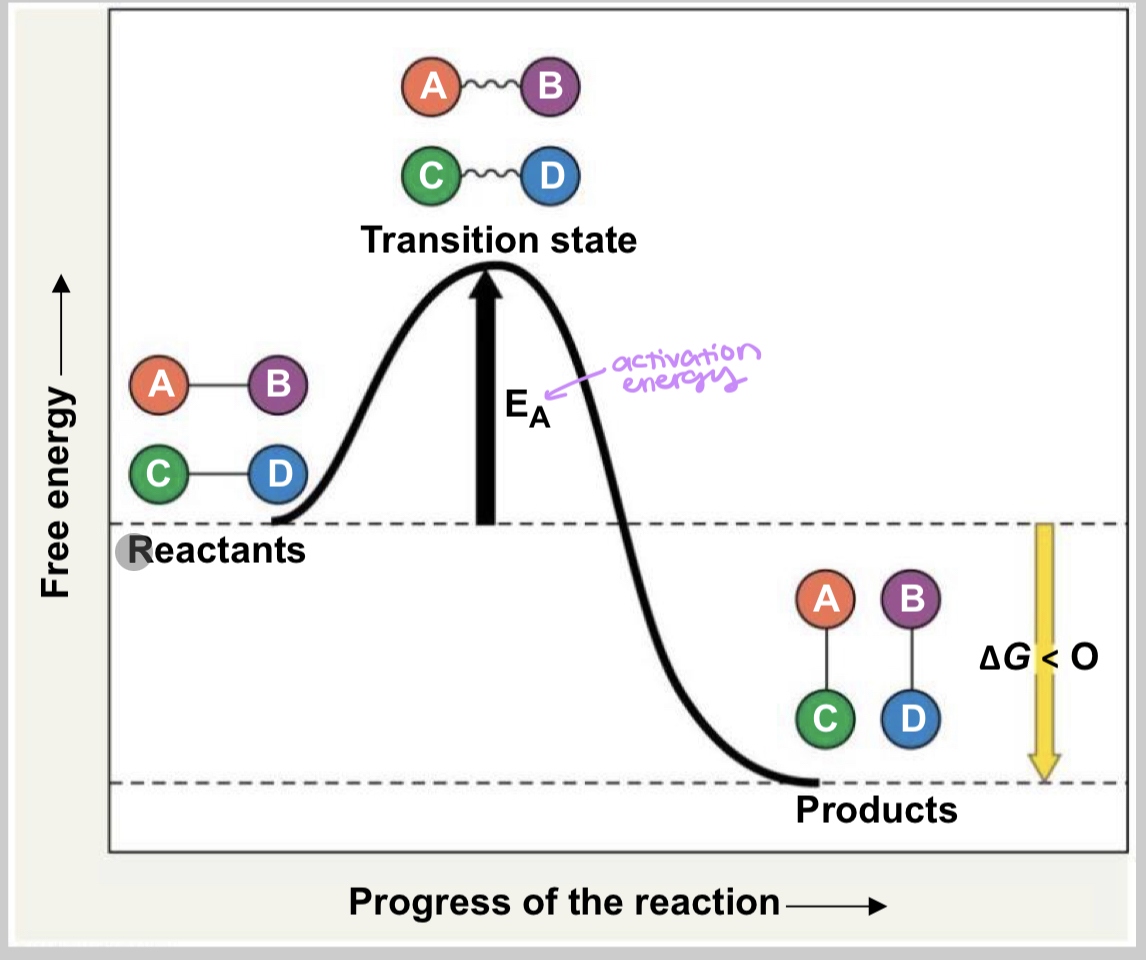
what is activation energy (EA)?
The minimum amount of energy needed to cause specific reaction or process; often in the form of thermal energy that the reactant molecules absorb from surroundings
what effect does an enzyme have on EA?
an enzyme makes the EA lower
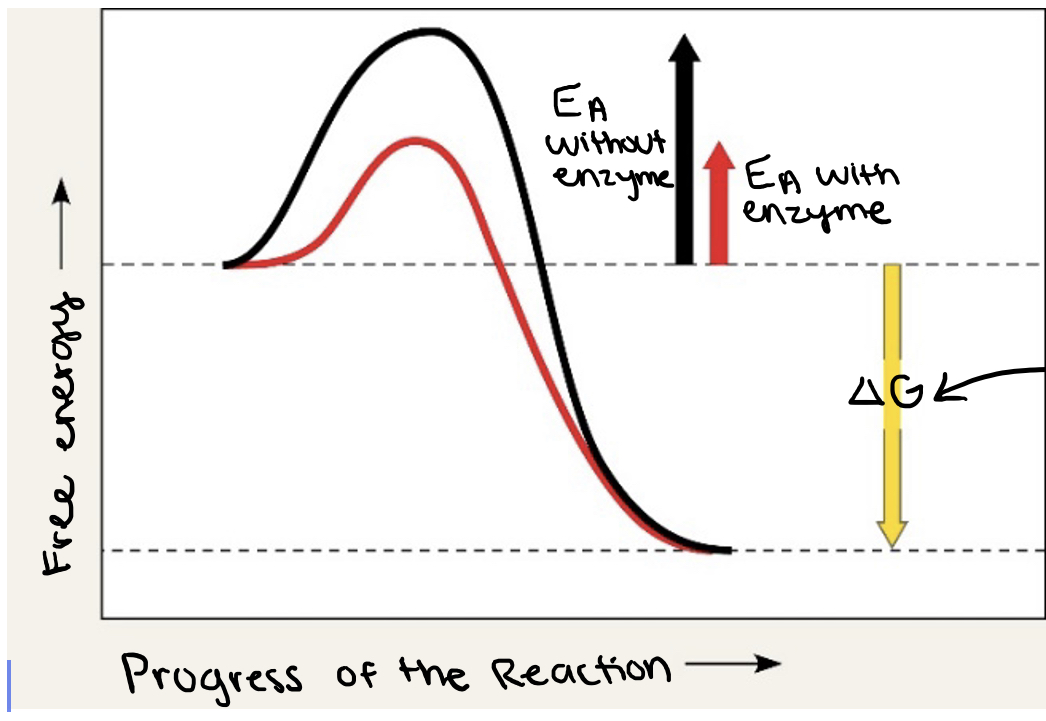
label ΔG. Is it positive or negative?
negative
How is ΔG affected by the enzyme?
unaffected
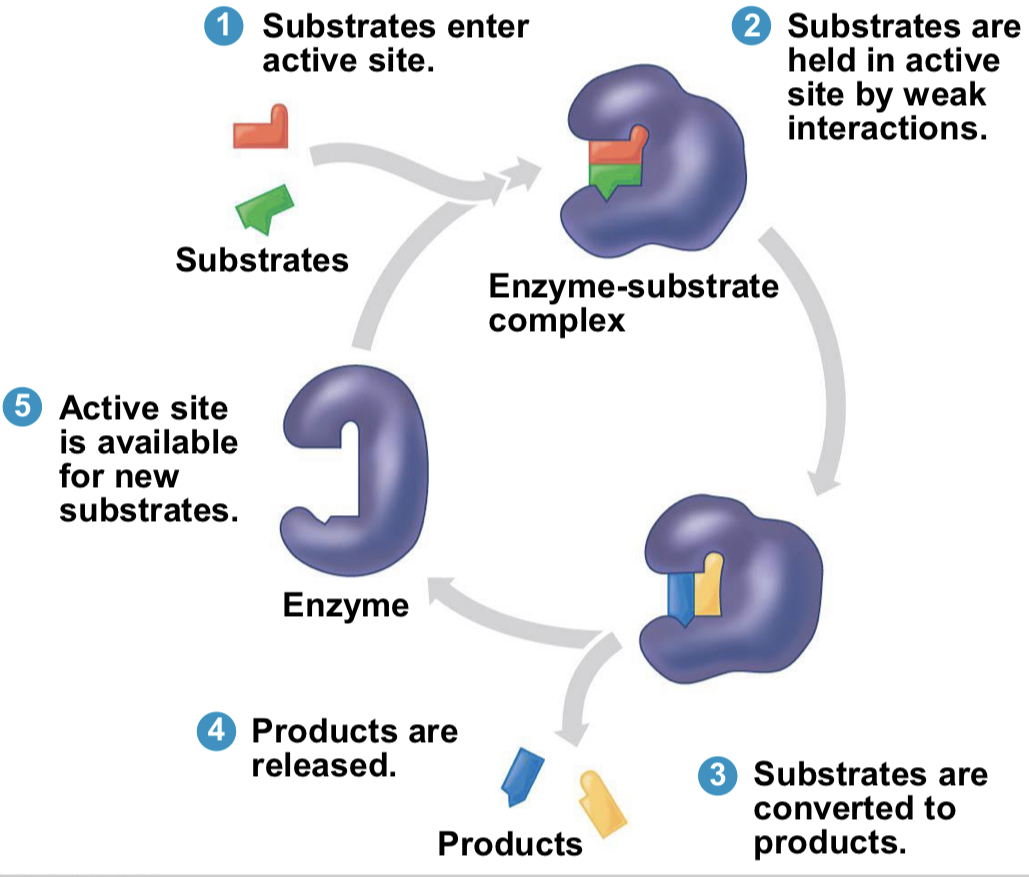
what is the substrate?
the reactant the enzyme acts on

A
total energy in presence if enzyme
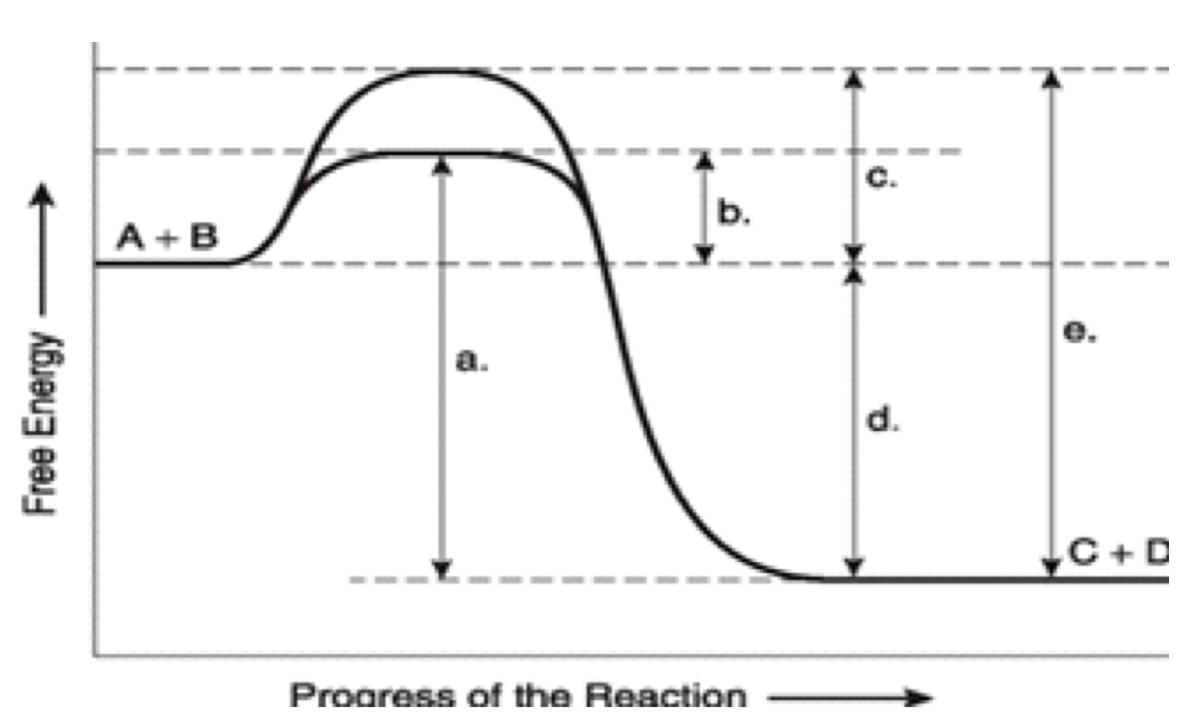
B
EA with enzyme
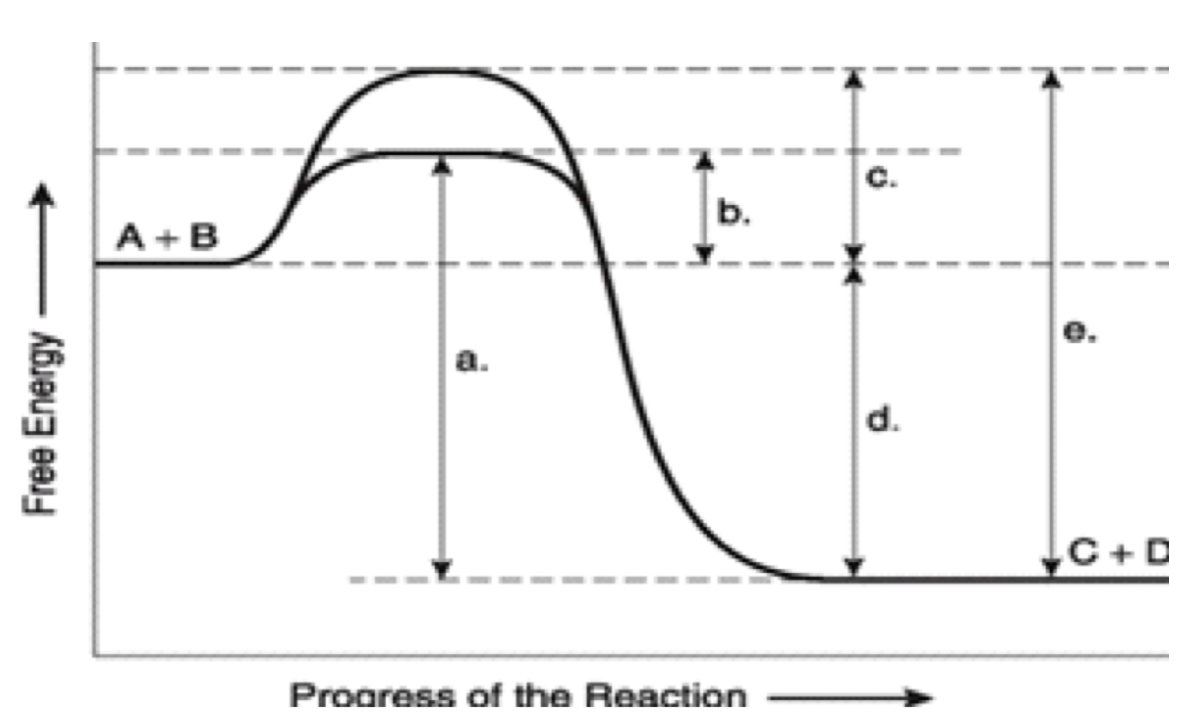
C
activation energy without enzyme
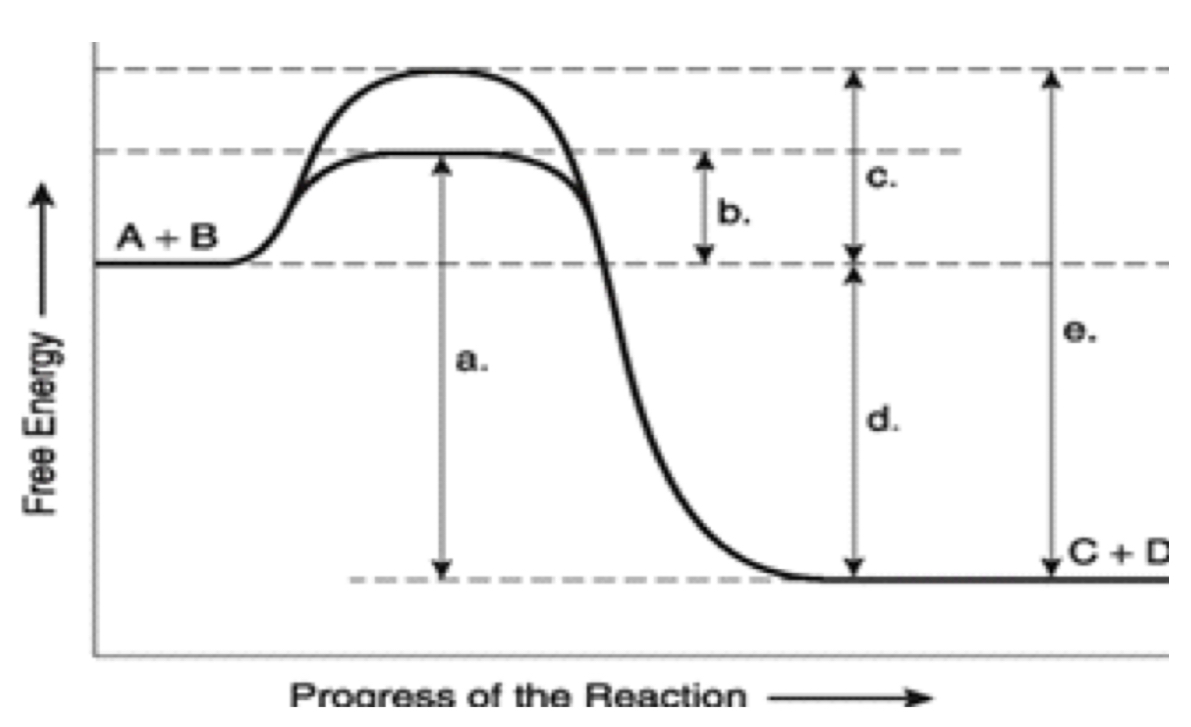
D
ΔG (unaffected by enzyme)
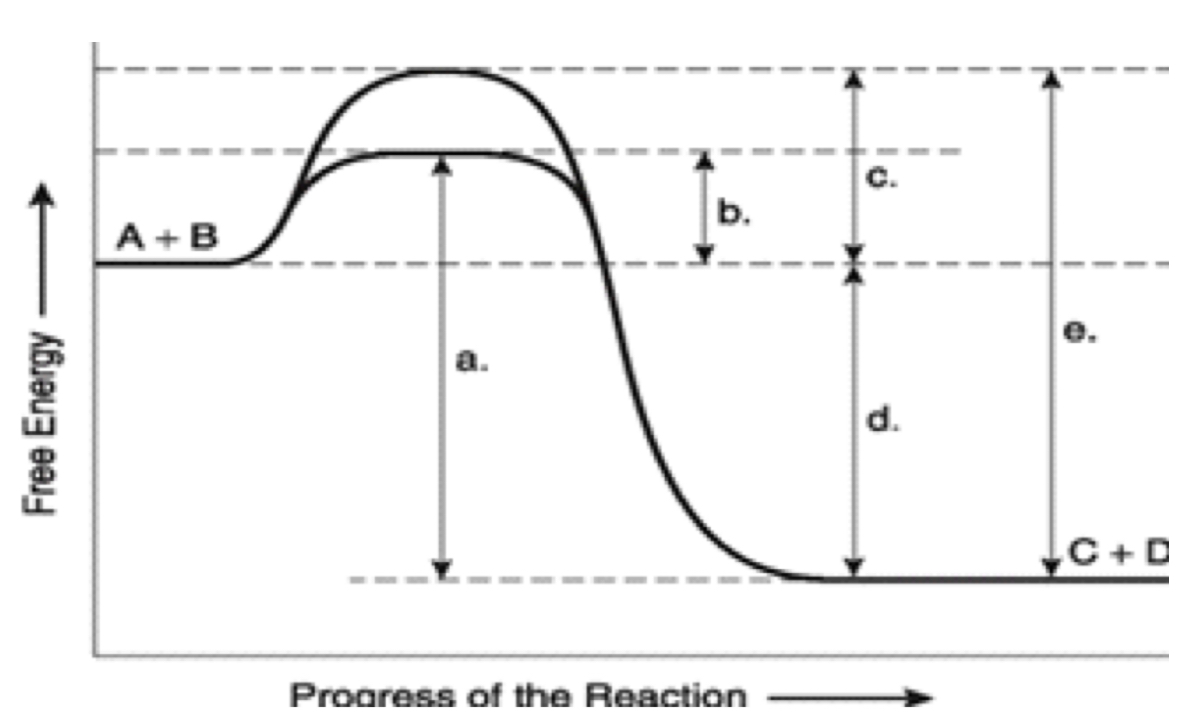
E
Total energy without enzyme
enzyme
catalytic protein
active site
area on enzyme where substrate fits
cofactors
nonprotein helpers for enzyme catalytic activity, often for chemical processes like electron transfers that cannot easily be carried out by amino acids in proteins
may be bound tightly rot the enzyme as permanent residents or bind loosely and reversible along with substrate
coenzyme
organic molecule serving as a cofactor (ex: many vitamins are coenzymes in metabolic reactions)
how does ATP couple reactions?
Coupling reactions in biological systems refer to the coordination of chemical reactions in which the energy released from one reaction is used to drive another reaction. These coupled reactions are essential in various cellular processes to maintain the energy balance and perform work within living organisms.
The role of ATP (adenosine triphosphate) in coupling reactions is fundamental. ATP acts as an energy carrier molecule in cells. It contains high-energy phosphate bonds that, when broken (hydrolyzed), release energy. This released energy is utilized to power endergonic (energy-absorbing) reactions in cells, making them energetically favorable.
energy coupling: use of exergonic process to drive endergonic one
competitive inhibitors
bind to the active site of an enzyme, competing with the substrate
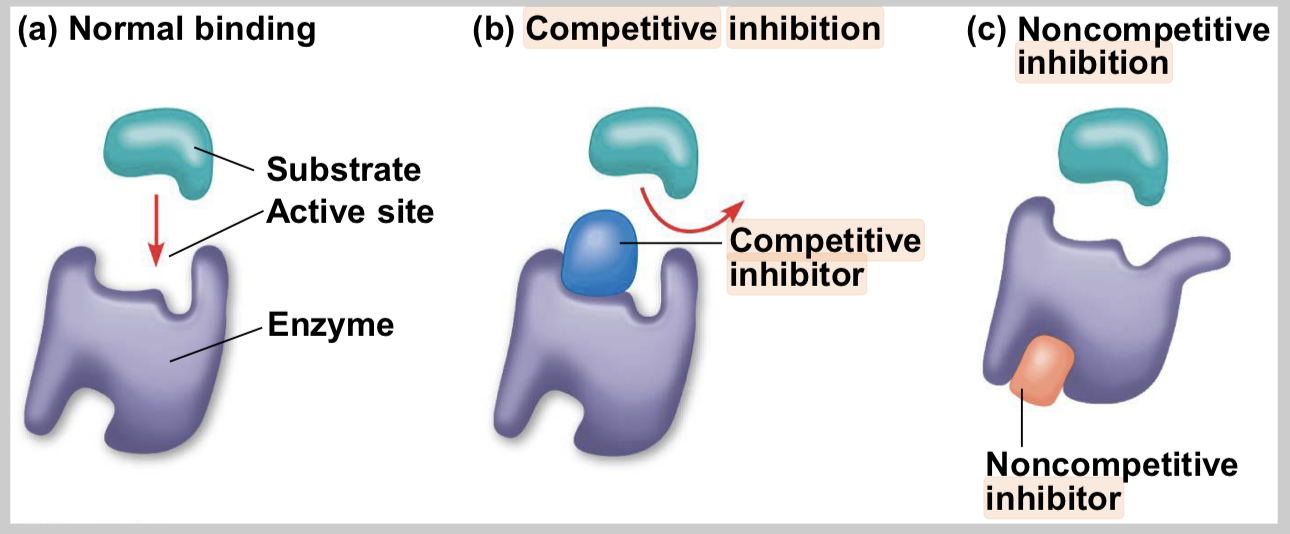
noncompetitive inhibitors
bind to another part of an enzyme, causing the enzyme to change shape and making the active site less effective
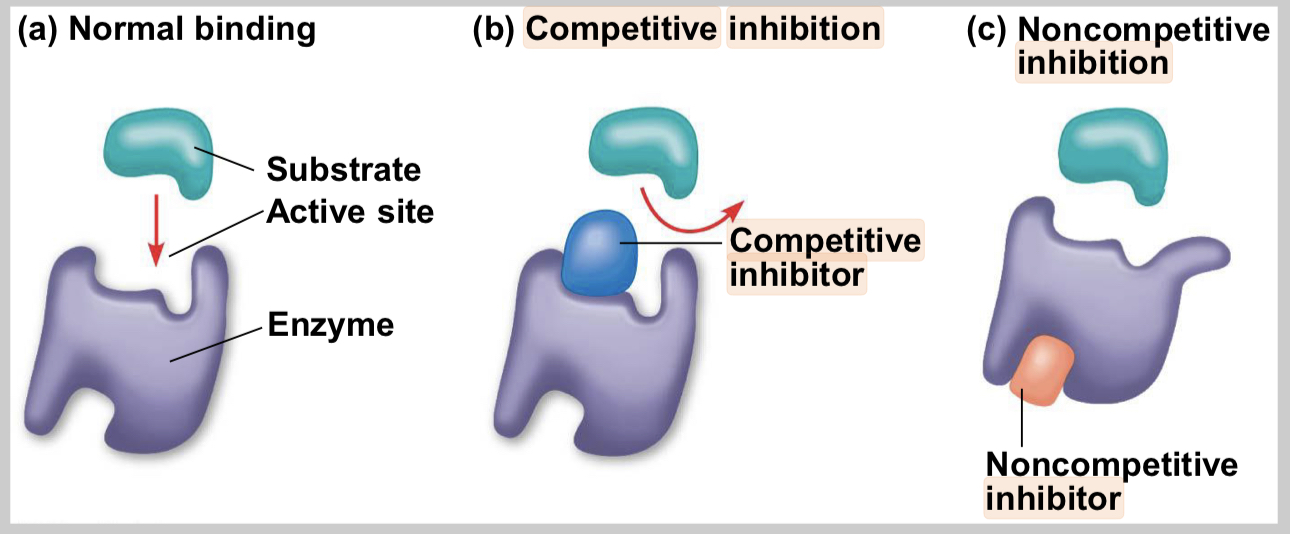
examples of inhibitors
toxins, poisons, pesticides, antibiotics
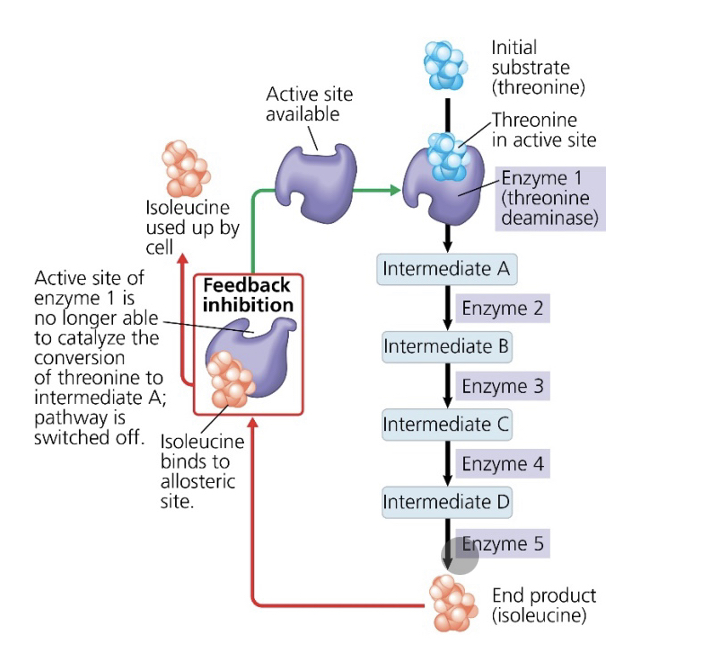
what is the substrate that initiates this metabolic pathway?
threonine
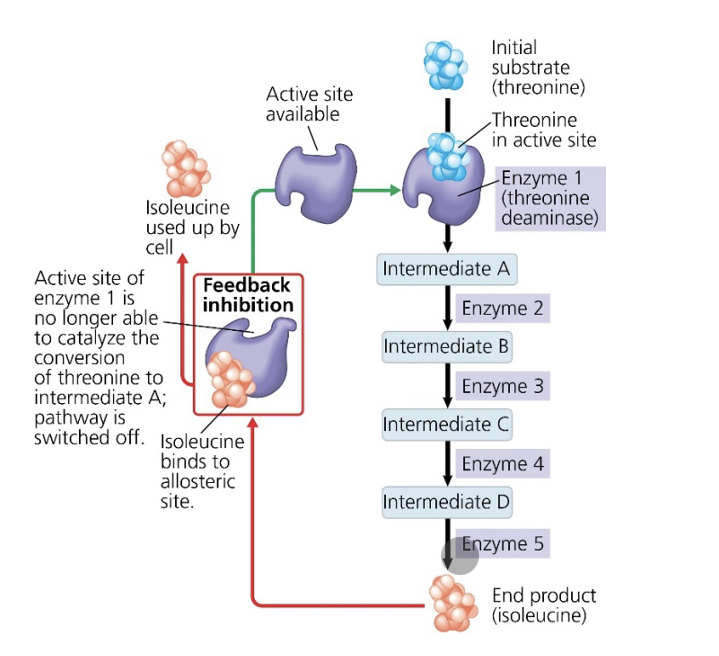
what is the inhibitor molecule?
isoleucine
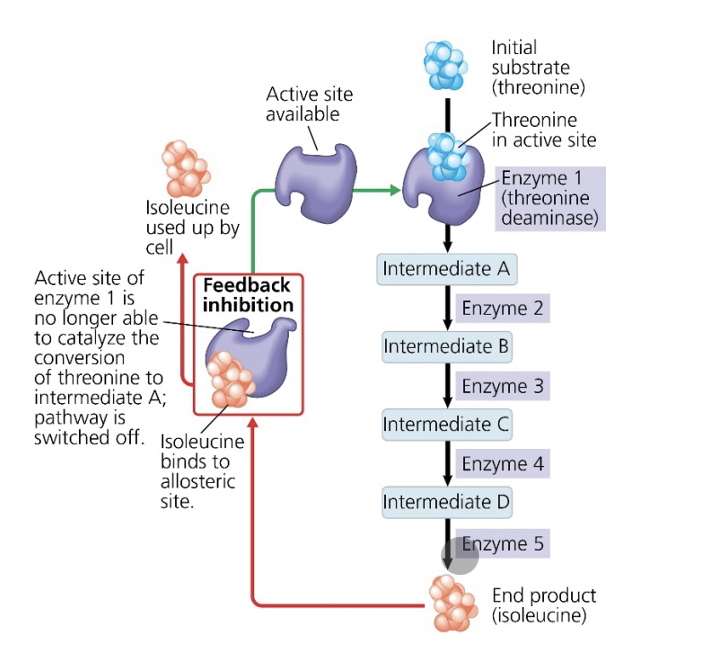
what type of inhibitor is it?
allosteric inhibitor

what type of metabolic control is this?
negative feedback
what does it mean to be spontaneous? which type of metabolic pathway is spontaneous?
occurs without energy input; they can happen quickly or slowly
catabolic; must increase entropy of universe; a process is spontaneous and can perform work only when it is moving towards equilibrium
spontaneous processes are energetically favorable
when is change in G negative and positive?
it is negative in catabolic reactions (releases energy) and positive in anabolic reactions (requires energy)
heat (thermal energy)
kinetic energy + random movement of atoms or molecules
chemical energy
potential energy for release in chemical reaction
thermodynamics
study of energy transformations
isolated vs open system
isolated: such as that approximated by liquid in a thermos, is unable to exchange energy or matter with its surroundings
open system: energy and matter can be transferred between the system and its surroundings (ex: organisms)
change in free energy equation
The change in free energy (∆G) during a process is related to the change in enthalpy, or change in total energy (∆H), change in entropy (∆S), and temperature in Kelvin units (T)
∆G = ∆H - T∆S

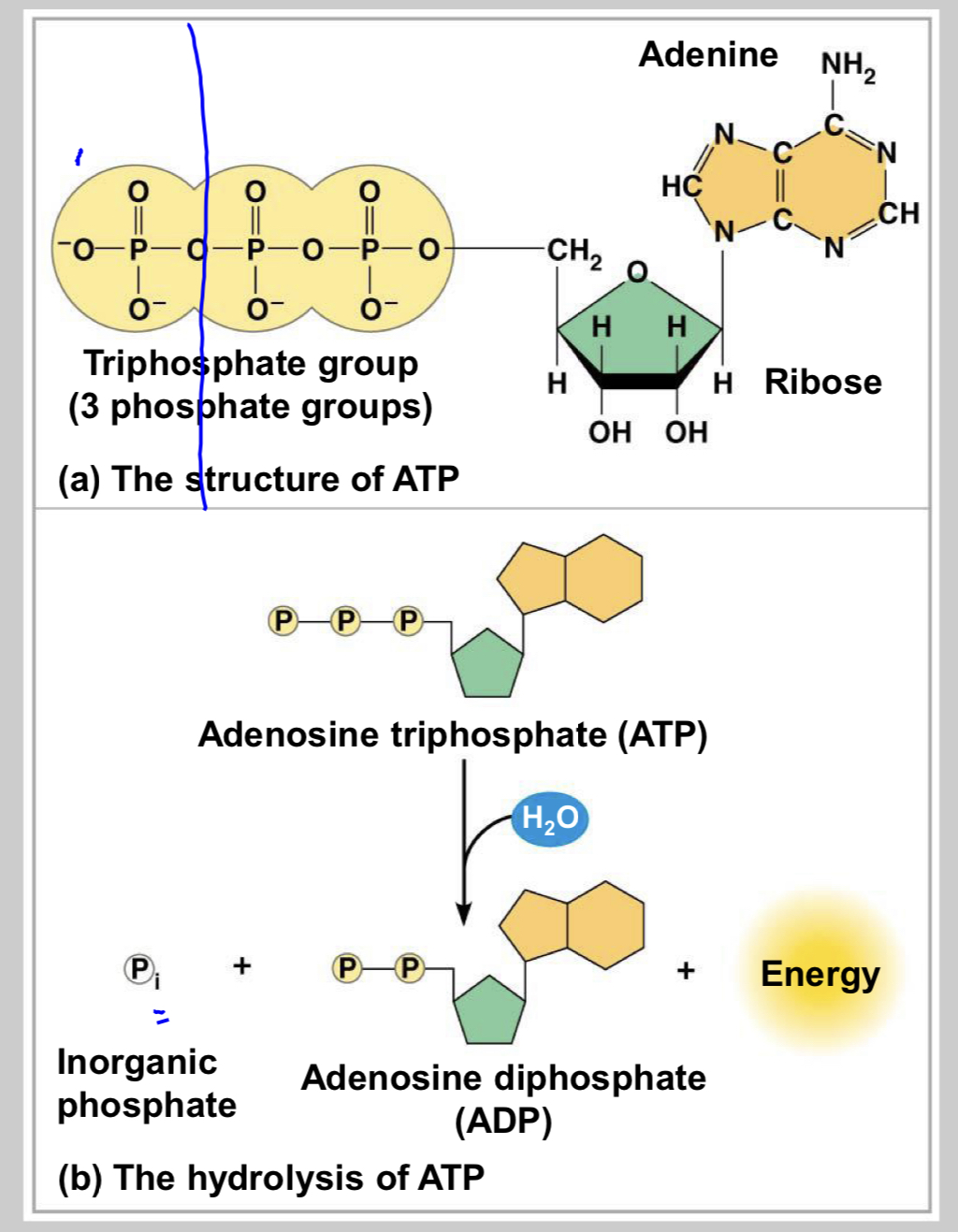
interpret image
ATP structure
ribose (sugar), adenine (nitrogenous base), three phosphate groups
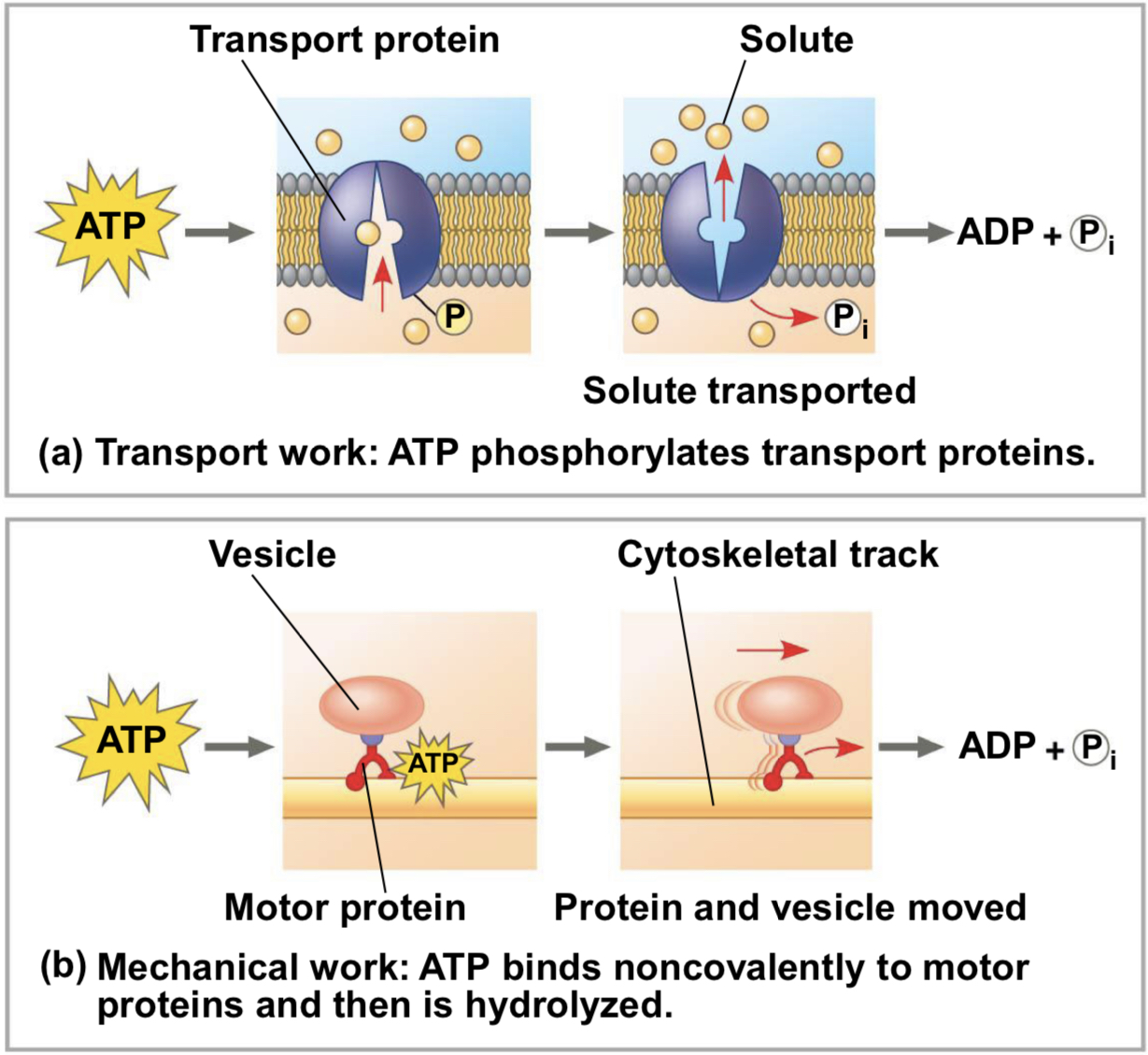
three types of cellular work
mechanical, transport, chemical
phosphorylation
ATP drives endergonic reactions by phosphorylation, transferring a phosphate group to some other molecule, such as a reactant
recipient molecule now called phosphorylated intermediate
ATP hydrolysis
the energy from the exergonic reaction of ATP hydrolysis can be used to drive an endergonic reaction
▪ Overall, the coupled reactions are exergonic
how does the enzyme lower EA barrier?
orienting substrates correctly
straining substrate bonds
providing a favorable microenvironment
covalently bonding to substrate
allosteric regulation
may either inhibit or stimulate an enzymes activity
allosteric regulation occurs when a regulatory molecule binds to a protein at one site and affects the proteins function at another side
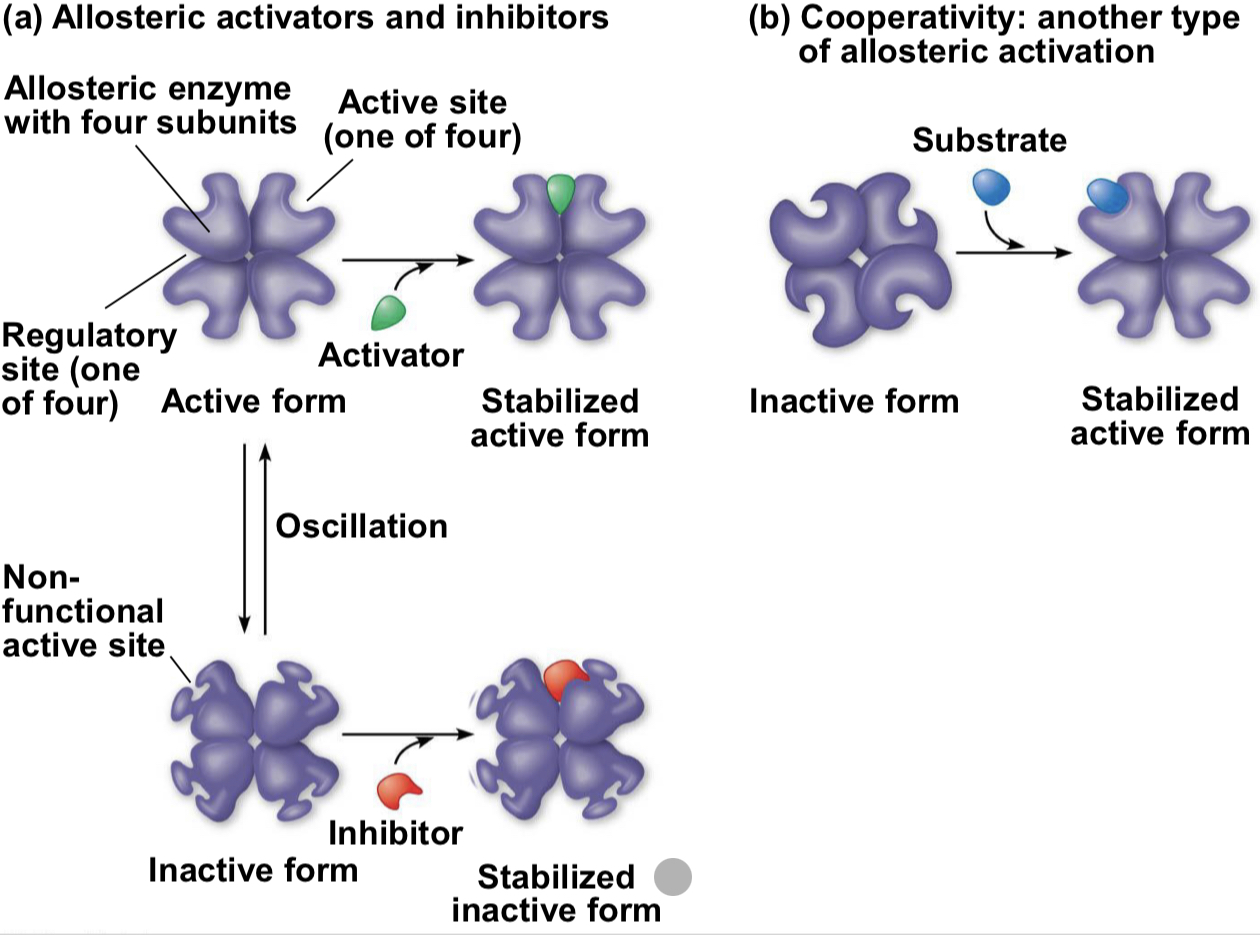
cooperativity
form of allosteric regulation that can amplify enzyme activity
one substrate mol primes an enzyme to act on additional substrate moles more readily
cooperativity is allosteric bc binding by a substrate to one active site affects catalysis in a different active site
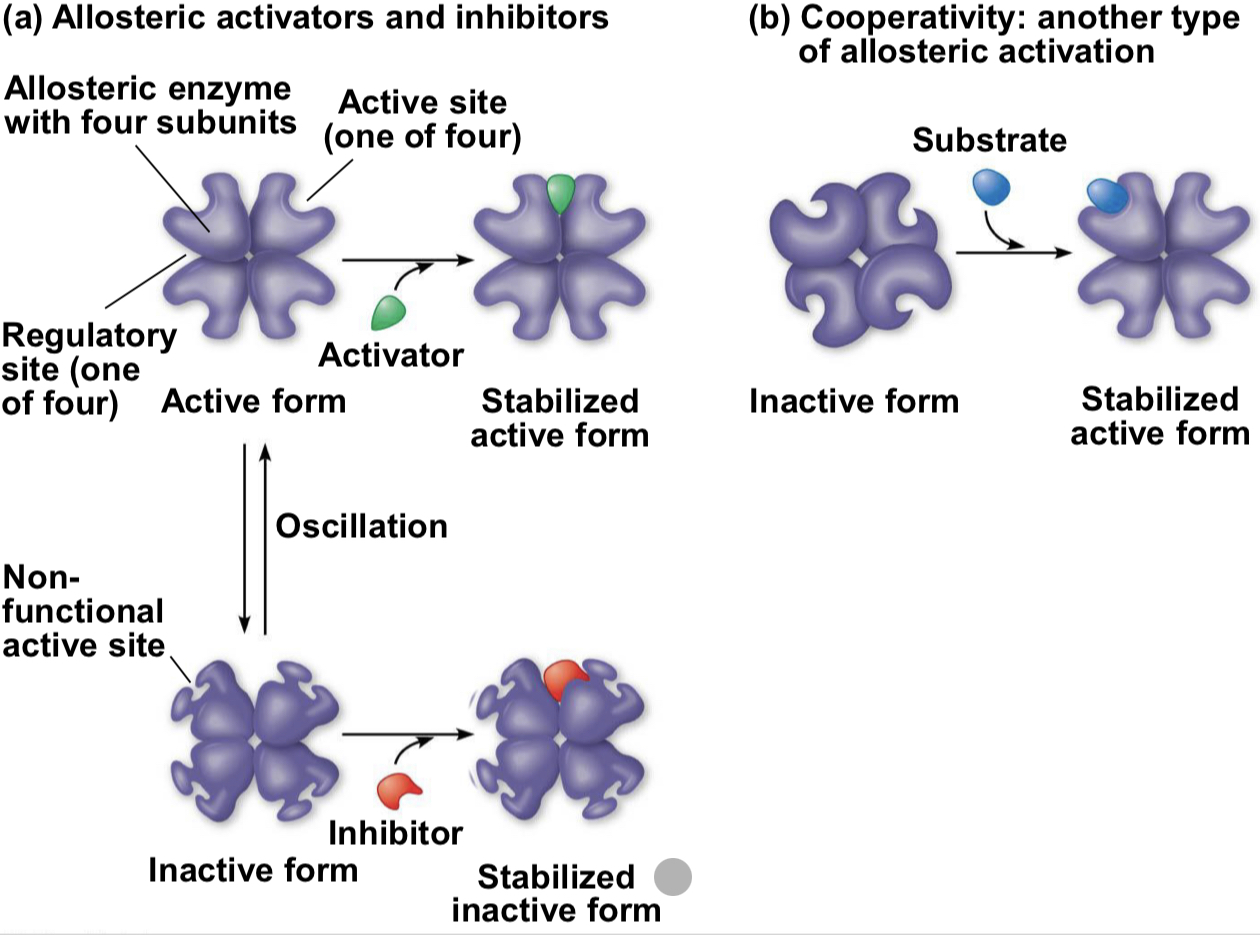
noncompetitive inhibition vs allosteric inhibition
Allosteric Inhibition: may either inhibit or stimulate an enzyme’s activity, occurs when a regulatory molecule binds to a protein at one site and affects the protein’s function at another site
Noncompetitive Inhibition: bind to another part of an enzyme, causing the enzyme to change shape and making the active site less effective
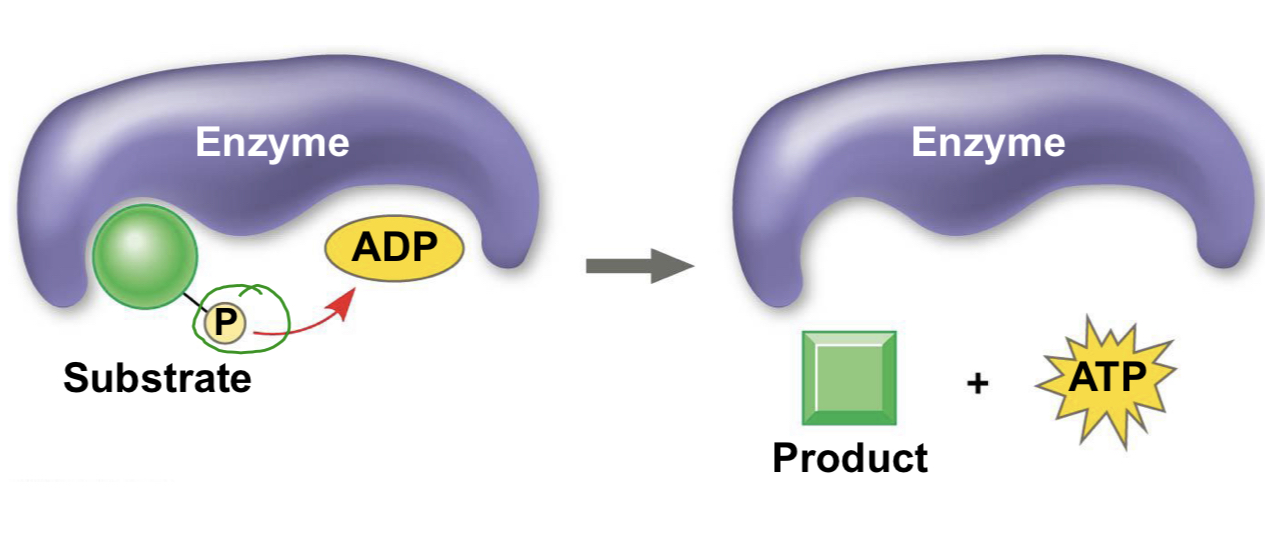
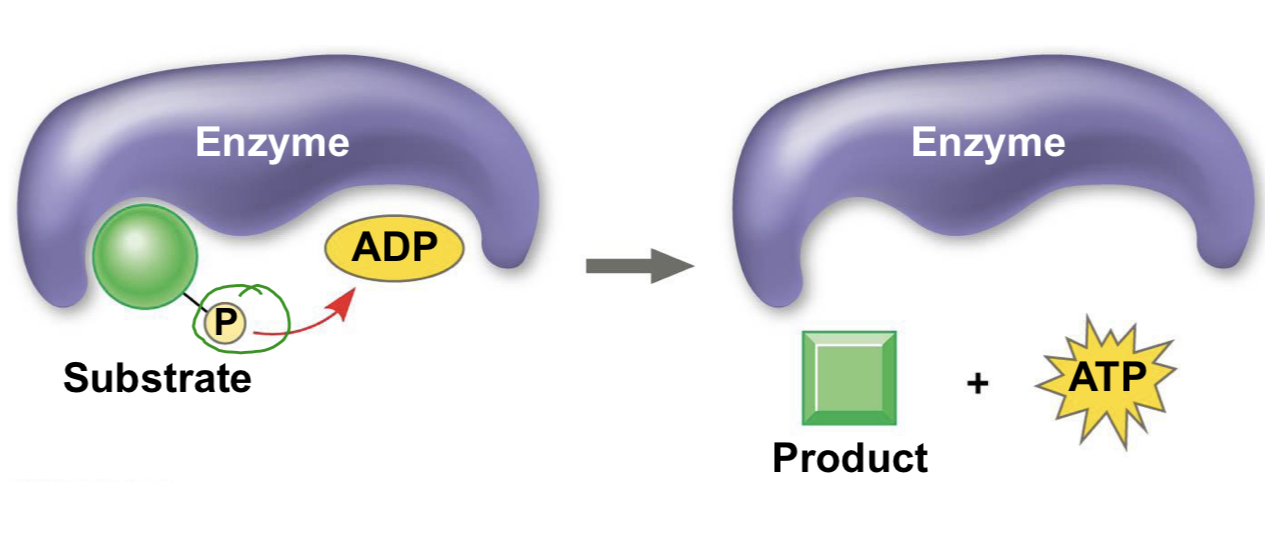
substrate-level phosphorylation
A mode of ATP synthesis in which an enzyme transfers a phosphate group from a substrate molecule to ADP rather than addicting an inorganic phosphate to ADP as in oxidative phosphorylation. This form creates less ATP than oxidative phosphorylation
oxidative phosphorylation
A mode of ATP synthesis in which energy derived from the redox reactions of an electron transport chain is used; creates more ATP than substrate-level phosphorylation
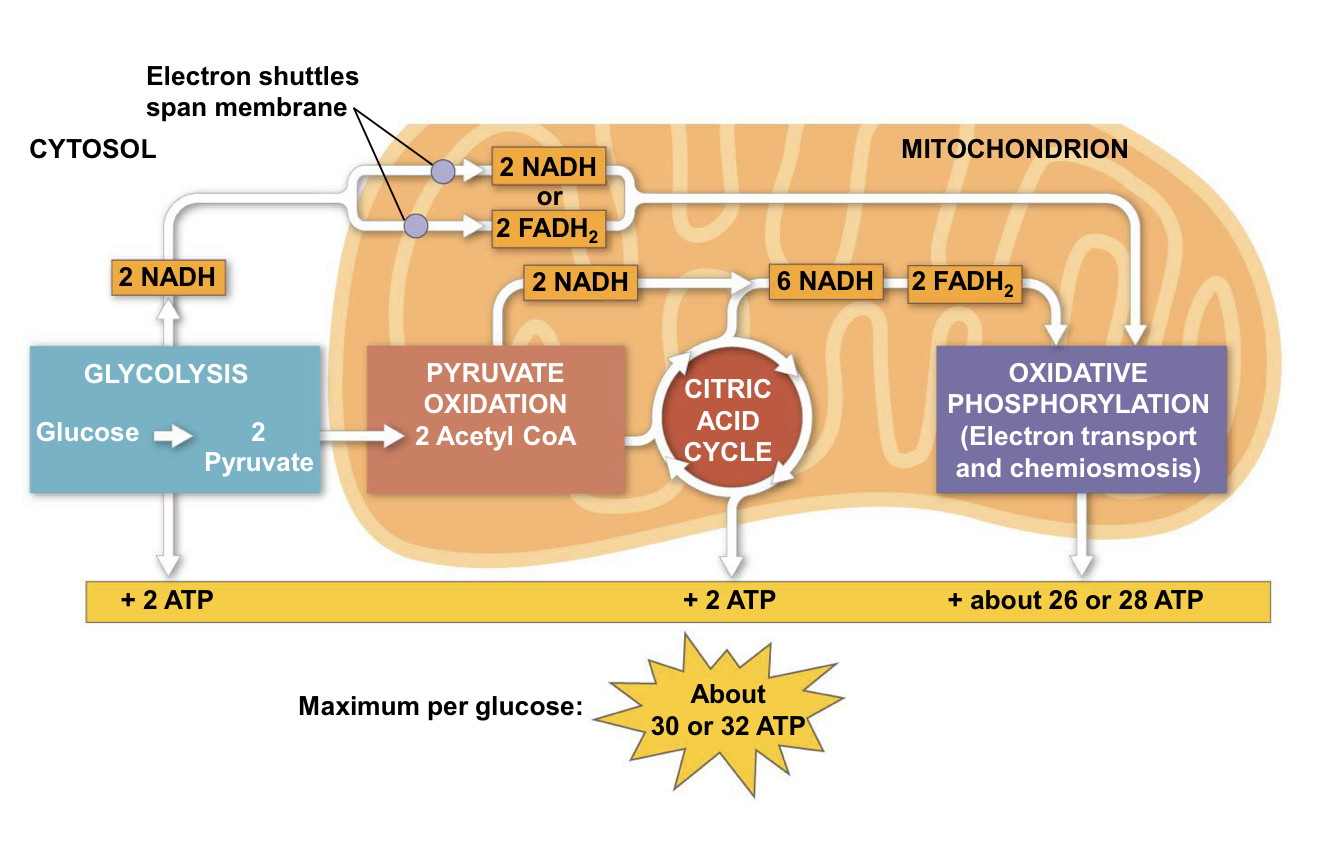
4 stages of cellular respiration
glycolysis
pyruvate oxidation
the krebs cycle/citric acid cycle
electron transport chain
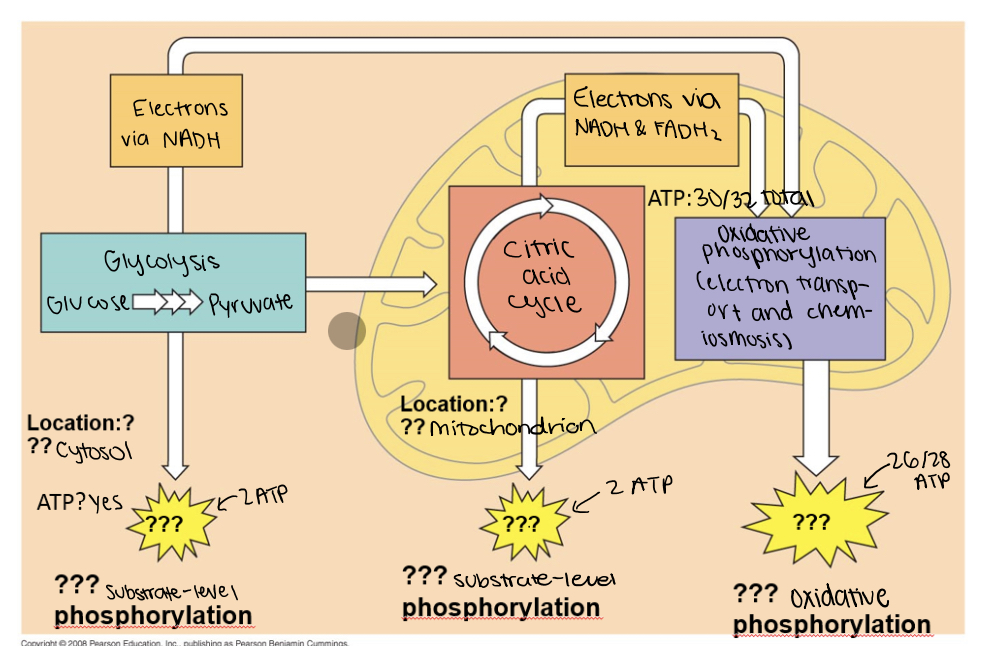
glycolysis
location: cytoplasm
starts with: breaking down glucose into two molecules of pyruvate (2 3 carbon compounds)
produces: pyruvate, 2 ATP
produces ATP through: substrate-level phosphorylation
oxidation of pyruvate
location: mitochondria matrix
starts with: pyruvate first converted into acetyl coenzyme A (acetyl CoA)
produces: CO2, NADH, acetyl CoA (2c)
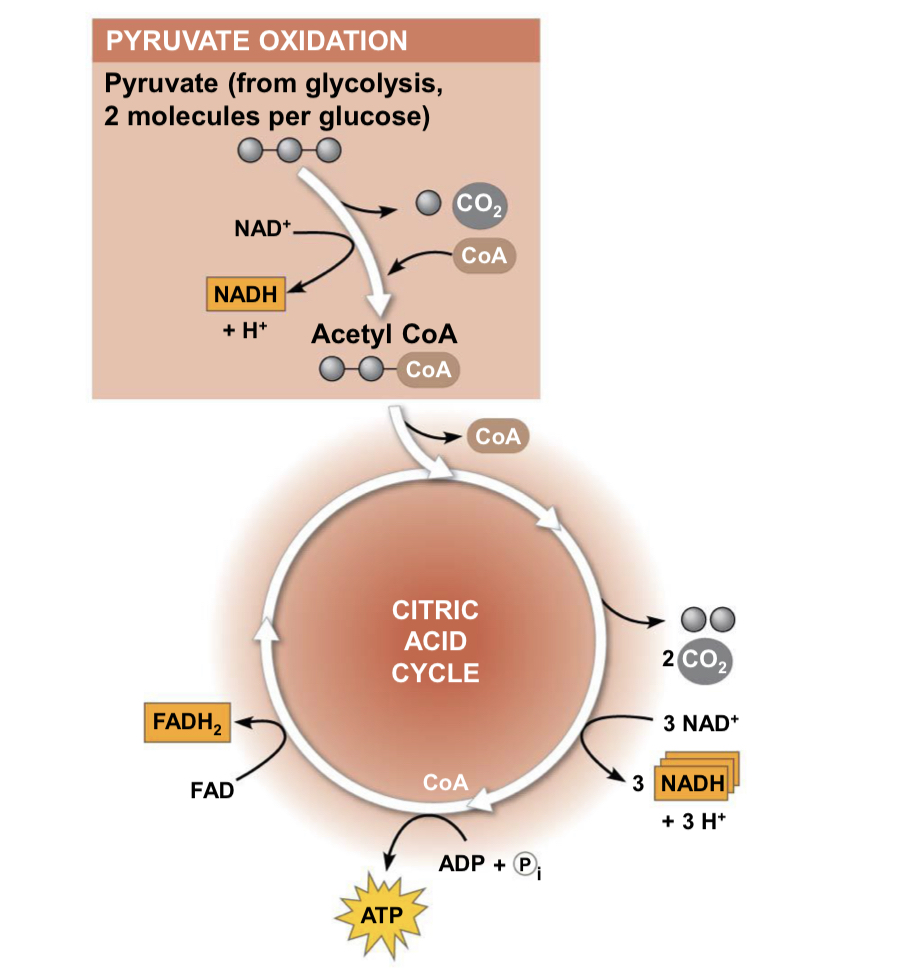
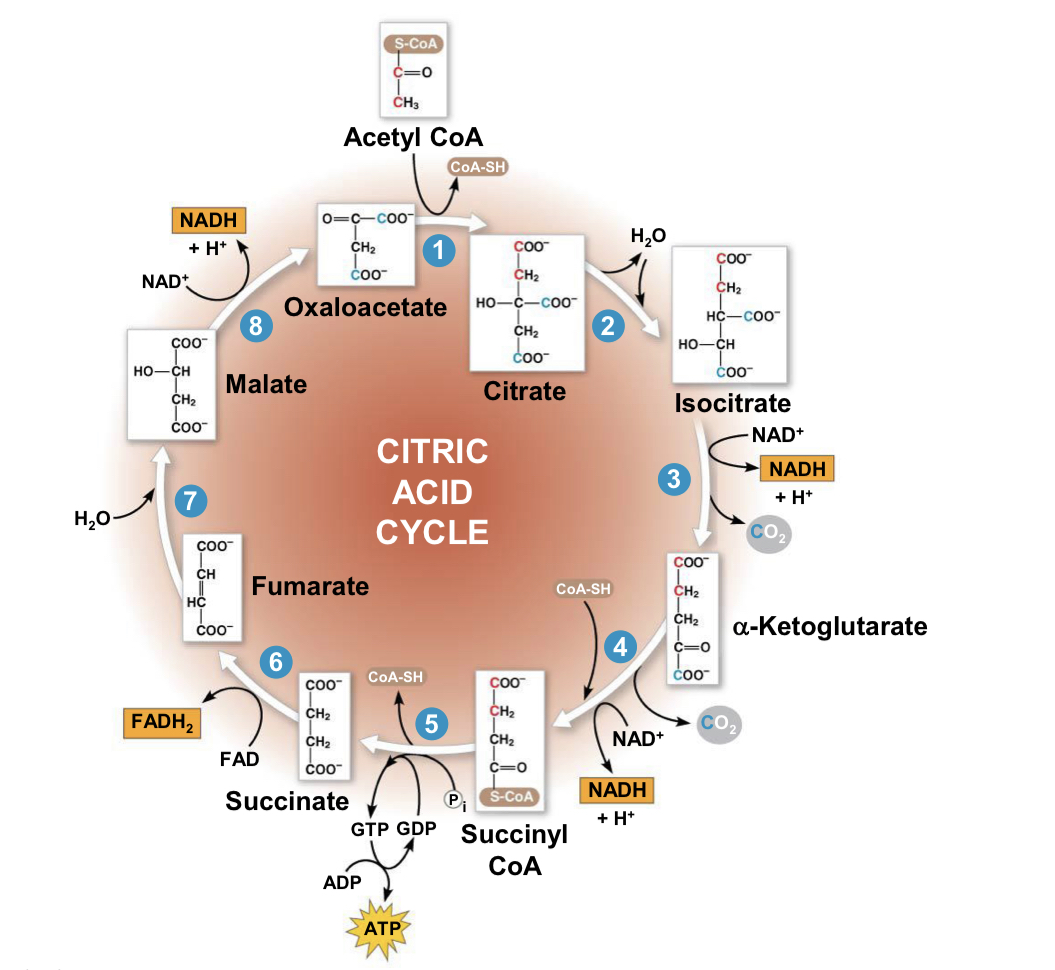
krebs cycle/citric acid cycle
location: mitochondria matrix
starts with: two carbons (red) enter in the relatively reduced form of an acetyl group, the acetyl group of acetyl CoA joins the cycle by combining with the compound oxaloacetate, forming citrate
produces: (1 ATP, 3 NADH, 1 FADH2 per turn)x2 for total
produces ATP through: substrate-level phosphorylation
major function: completes breakdown of glucose
the roles of NAD+ and FAD in respiration: electron acceptors that carry them to electron transport chain

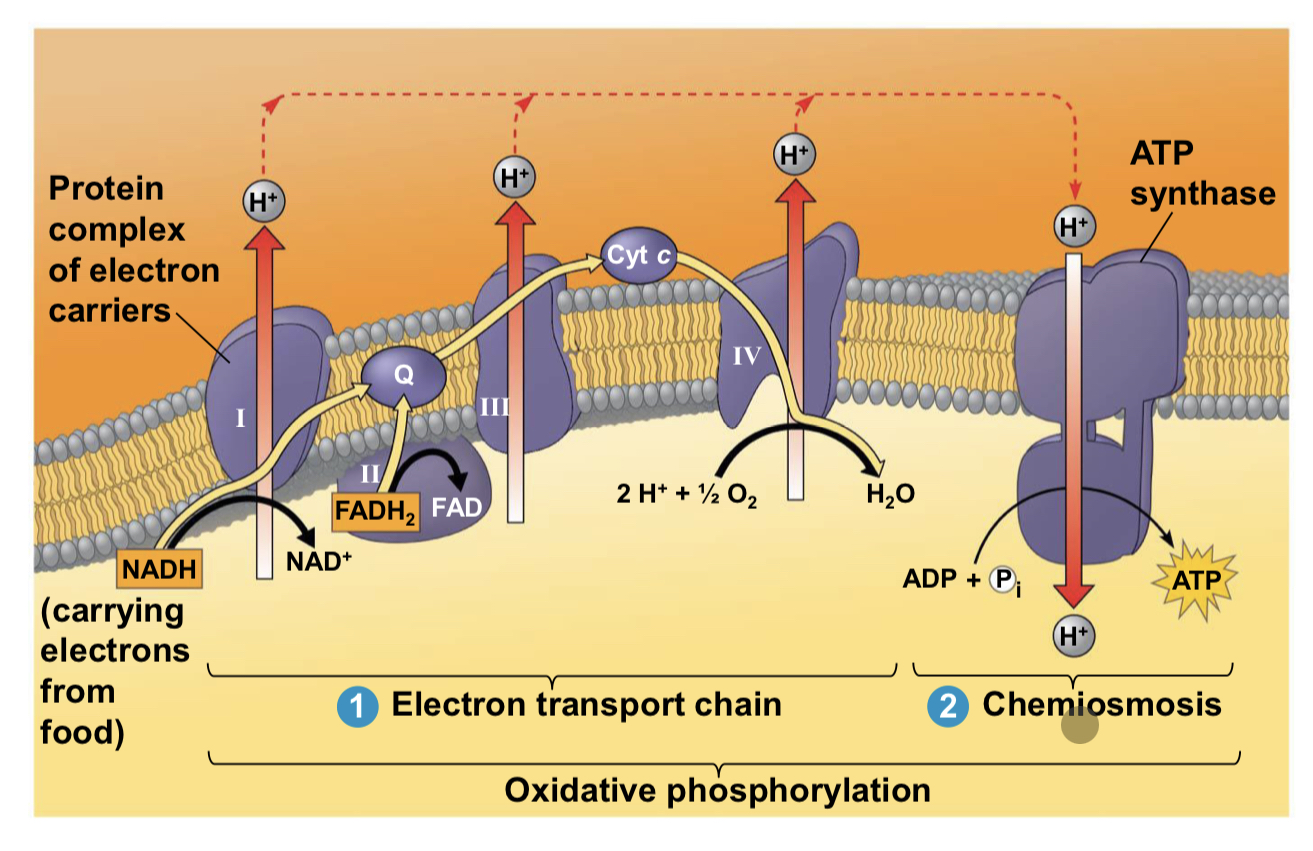
electron transport chain
location: mitochondria inner membrane
starts with: donation of electrons by NADH (nicotinamide adenine dinucleotide) and FADH2 (flavin adenine dinucleotide) to the first protein complex in the chain, known as Complex I or NADH dehydrogenase
produces: 26/28 ATP, NAD+, FAD, water
produces ATP through: oxidative phosphorylation
How is Glycolysis regulated? Name of the enzyme, where does it act and regulate and what does it control? When does it regulate?
Glycolysis is regulated by the enzyme, phosphofructokinase. It is in the cytoplasm. It regulates glycolysis through allosteric inhibition, and through doing so regulates the rate of glycolysis. It slows down the process when there is excess ATP and speeds the process back up when there is not enough ATP.
what is the final electron acceptor in the electron transport chain?
oxygen
Describe the role of the Electron Transport Chain. What happens to the electrons and H+?
It produces the most ATP in cellular respiration. The electrons and H+ move through channels down the concentration gradient and produce ATP.
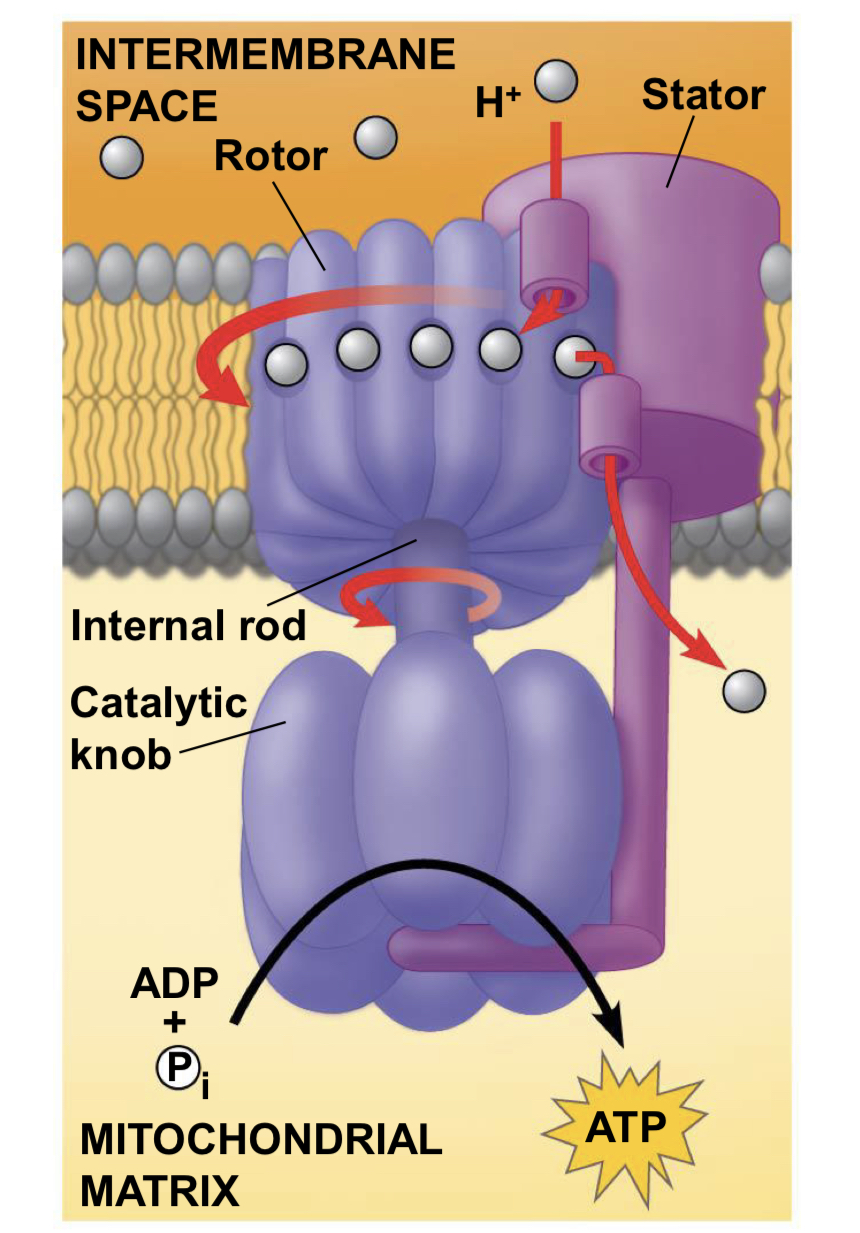
what is chemiosmosis and how is it generated?
chemiosmosis is the use of energy in a H+ gradient to drive cellular work. It is generated by the flow of electrons down the electron transport chain.
explain why respiration is considered exergonic
it is accompanied by the release of ATP
alcoholic fermentation converts glucose to
ethanol and carbon dioxide
alcoholic fermentation is utilized by what organisms
yeast, bacteria
lactic acid fermentation converts glucose to what
lactic acid
lactic acid fermentation is utilized by what organisms?
yogurt bacteria, human muscle cells
Which process produces more ATP Aerobic, Anaerobic respiration or fermentation?
aerobic respiration
where does substrate phosphorylation take place?
glycolysis, citric acid cycle
where does oxidative phosphorylation take place?
electron transport chain, chemiosmosis
difference between two phosphorylations
Substrate-level phosphorylation occurs when enzymes remove a high-energy phosphate from a substrate and directly transfer it to ADP. Oxidative phosphorylation occurs when electrons move through an ETC and proficient a proton-motive force that drives ATP synthesis. Both processes produce ATP from ADP and Pi.
how is carbon dioxide made, which cycle(s) do you see this happening?
carbon dioxide is made through the breakdown of pyruvate; occurs in citric acid cycle
what happens to the carbon that is released from pyruvate that becomes Acetyl CoA? where else in the central metabolic pathway do you see this?
the carbon that is released from pyruvate is released as carbon dioxide
this also occurs in the citric acid cycle
what is the outcome of ATP, NADH, in glycolysis and citric acid cycle?
glycolysis: 2 ATP, 2 NADH
citric acid cycle: 2 ATP, 6 NADH (TOTAL)
how many ATPs are made in ETP? What happens to the NADH - oxidized or reduced?
ETC makes 26/28 ATP. The NADH is oxidized to NAD+
which enzyme helps in making ATP? where is it located, how many protons are needed for one ATP and where are the protons?
ATP synthase
located in mitochondria, and about 3.33 protons are needed for one ATP
the protons are also located in the mitochondria inner membrane space
how is water made in the ETC? who is the electron acceptor? which part of the hydrogen is forming water (electrons or protons)?
water is made by adding H+ ions to O2
the electron acceptor is oxygen
the part of hydrogen forming water is the protons
fermentation
partial degradation of sugars that occurs without O2
aerobic respiration
(aka cellular respiration) consumes organic molecules and O2 and yields ATP
cellular respiration includes both aerobic and anaerobic respiration but is often used to refer to aerobic respiration
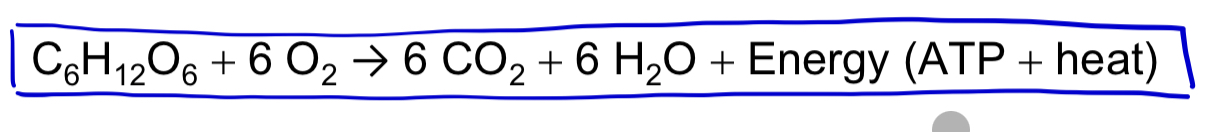
anaerobic respiration
similar to aerobic respiration but consumes compounds other than O2
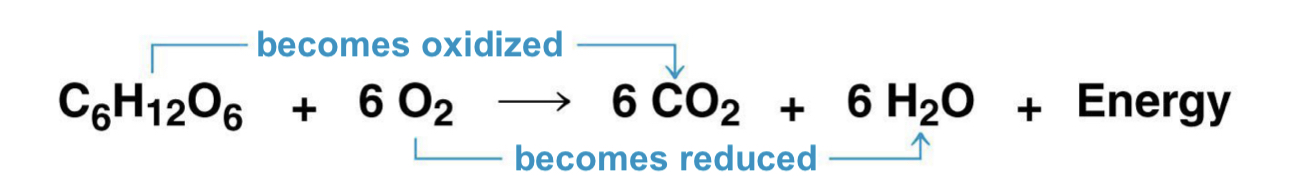
redox reactions
oxidation and reduction
transfer or electrons during chemical reactions releases energy stored in organic moles
this released energy is used to synthesize ATP

reducing agent
electron donor
electron acceptor
oxidizing agent
during cellular respiration, the fuel (such as glucose) is ___, and O2 is ___
oxidized, reduced
function of NAD+ in cellular respiration
electron acceptor, oxidizing agent
each NADH (reduced form of NAD+ represents stored energy that is tapped to synthesize ATP)
reduction is to _ as oxidation is to _
hydrogenation, dehydration
in the ETC, __ pulls down chain in an energy yielding tumble
O2, energy yielded used to regenerate ATP
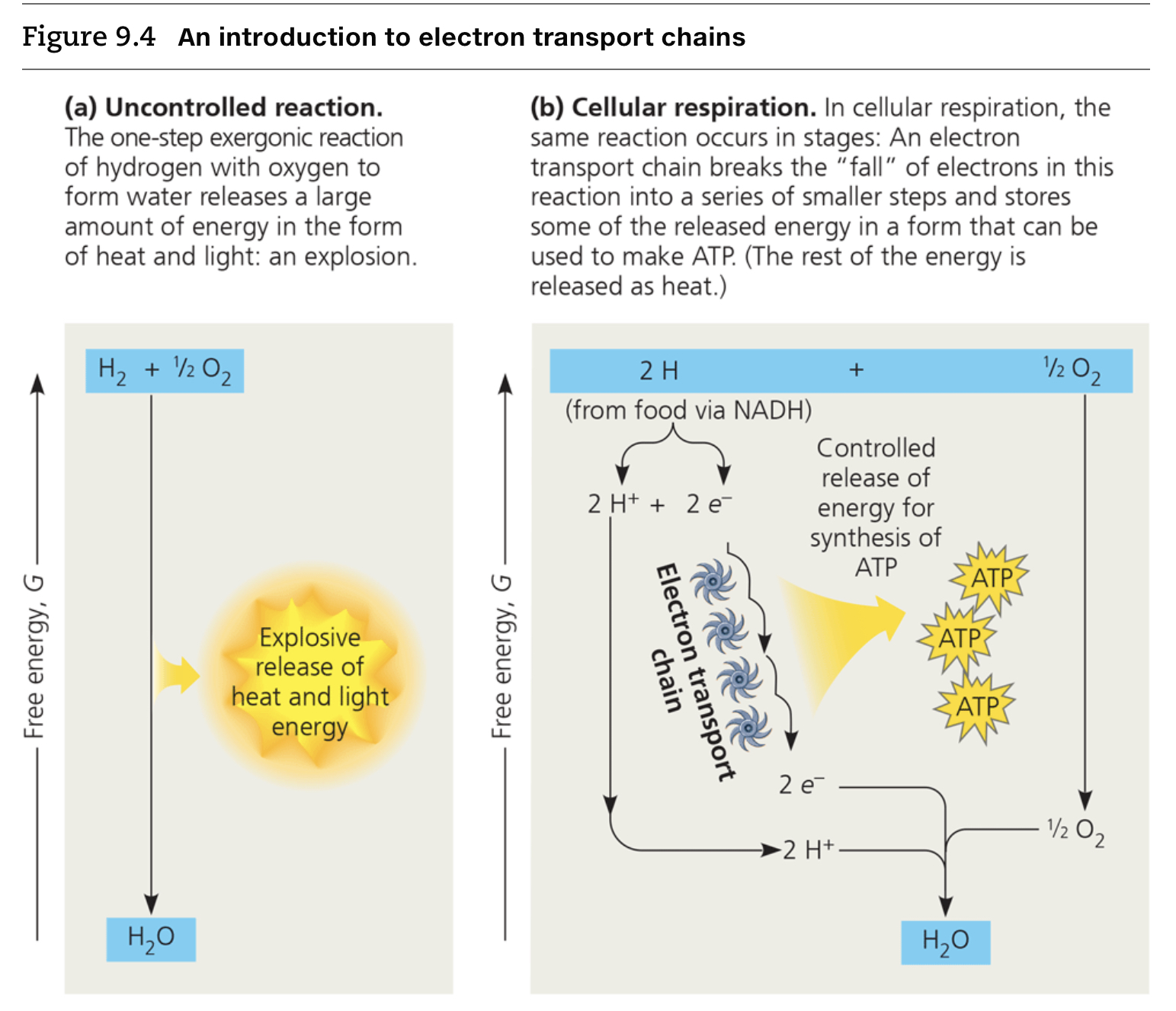
substrate level phosphorylation
This mode of ATP synthesis occurs when an enzyme transfers a phosphate group from a substrate molecule to ADP, rather than adding an inorganic phosphate to ADP as in oxidative phosphorylation.
true or false: glycolysis only occurs if O2 is present
false
glycolysis: energy investment phase vs energy payoff phase
energy investment: cell spends ATP
energy payoff: ATP is produced by substrate-level phosphorylation and NAD+ is reduced to NADH by electrons released from oxidation of glucose
does pyruvate oxidation require O2?
yes
steps of citric acid cycle
acetyl group of acetyl CoA joins cycle by combining with oxaloacetate, forming citrate
next seven steps decompose citrate back to oxaloacetate, making the process a cycle
NADH and FADH2 produced by cycle relay electrons extracted from food to electron transport chain
free energy outcome in ETC
electrons drop in free energy as they go down the chain and are finally passed to O2, forming H2O
does the ETC chain generate ATP directly?
no
chemiosmosis steps
Electron transfer in the electron transport chain causes proteins to pump H+ from the mitochondrial matrix to the intermembrane space
▪ H+ then moves back across the membrane, passing through the protein complex, ATP synthase
▪ ATP synthase uses the exergonic flow of H+ to drive phosphorylation of ATP
▪ This is an example of chemiosmosis, the use of energy in a H+ gradient to drive cellular work
the energy stored in H+ gradient across membrane couples redox reactions of electron transport chain to ATP synthesis - this H+ gradient is called a proton-motive force
energy flow sequence in cellular respiration
glucose —> NADH —> electron transport chain —> proton-motive force —> ATP
can the ETC work without oxygen
no, without O2 the ETC will cease to operate - in that case glycolysis couples with anaerobic respiration or fermentation to make ATP
anaerobic respiration uses ETC with final electron acceptor other than O2 (like sulfate)
what phosphorylation is used by fermentation instead of an ETC
substrate-level phosphorylation
what are the steps in alcohol fermentation that convert pyruvate to ethanol (2 steps)?
-release of CO2
-production of ethanol
lactic acid fermentation
pyruvate reduced to NADH, forming lactate as end product with no release of CO2