topic 7- intro to transition metal complexes
1/34
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
35 Terms
what is a transition metal?
any element possessing a partially filled d-subshell in its common oxidation state(s)
eg. Sc is not a TM bc SC3+ has an empty d-subshell and Zn isn’t either bc Zn2+ has a full d-subshell
group 11 - Cu, Ag - bourderline bc it can form complexes with both filled and partially filled d subshells
what are the different transition series?
1st transition series - 3d - period 4
2nd transition series - 4d - period 5
3rd transition series - 5d - period 6
rules of electron configurations in the first row
in the transition ELEMENTS fill 4s before 3d (except copper and chromium) then rewrite in order of increasing value of n
in transition COMPOUNDS or COMPLEXES fill 3d before 4s bc they’re lower in energy in complexes
d^n configuration
the number of outer electrons present
number of outer s + d electrons
eg. Ni2+ is a “d^8 metal”
physical properties of transition metals
hard
ductile
malleable
high electrical conductivity
high thermal conductivity
(form coloured complexes)
solid state structure of TMs
all TMs except Mn, Zn, Cd + Hg adopt one of three typical metal structures
hexagonal close packed
face centred cubic
body centred cubic (not a close packed arrangement)
ligands
the atoms to molecules directly bonded to a central metal ion
coordination number
the number of direct points of attachment to the metal ion or bonds
teh number of donor atoms bound to a transition metal centre
source of colour in transition metal complexes
‘d-d’ transitions - electrons moving between d orbitals
L-M charge transfer - electrons originally from the ligand get excited to the metal - normally gives rise to absorbance in the visible region
M-L charge transfer - electrons originally from metal are excited to orbitals on ligand
which type occurs depends on the properties of the ligands and the metals themselves
diamagnetism
substances that contain only paired electrons
diamagnetic substances are repelled from a magnetic field
show a decrease in weight when placed into an applied magnetic field (using a guoy balance)
paramagnetism
substances that contain unpaired electrons
paramagnetic substances are attracted into a magnetic field
paramagnetic compounds show an increase in weight when placed into an applied magnetic field (using a guoy balance)
which effects are weaker? diamagnetic or paramagnetic?
diamagnetic effects re much weaker than paramagnetic effects
if the compound has unpaired electrons, paramagnetic effects dominate and the compound is attracted into the magnetic field
predicting effective magnetic moment
spin only formula
mu effective = square root n(n+2)
where n=the number of unpaired electrons in d orbitals
are high oxidation state complexes oxidising or reducing agents?
oxidising
are low oxidation state complexes oxidising or reducing agents?
reductants
why are high oxidation states on the left and low oxidation states on teh right?
ionisation potential gets bigger from left to right due to nuclear charge increasing so it is harder to remove an electron
would you expect CrO3 to be an oxidising or reducing agent?
+6 is chromiums maximum oxidation state so it can only be reduced therefore it’s and oxidising agent
paulings electroneutrality principle
the charge of a single atom can never be greater than one - if it is it needs to be spread about a wider molecule
the net charge of a molecule is close to neutrality - each atom in a stable molecule has a charge close to zero
examples of neutral two electron donors
NH3, CO, H2O, PR3, SR3
usually coordinate with lower oxidation state mental ions
examples of anionic two electron donors
CN-, Me-, Cl-, I-, H-
common with high oxidation states metal ions
examples of cationic two electron donors
NO+
very rare
will only coordinate to low oxidation states
“inner sphere” vs “outer sphere”
inner sphere - bound directly to metal centre i.e. L in [MLn]Xy
outer sphere - ligands associated with the ‘inner sphere’ complex i.e. X in [MLn]Xy
![<p>inner sphere - bound directly to metal centre i.e. L in [ML<sub>n</sub>]X<sub>y</sub></p><p>outer sphere - ligands associated with the ‘inner sphere’ complex i.e. X in [ML<sub>n</sub>]X<sub>y</sub> </p>](https://knowt-user-attachments.s3.amazonaws.com/bfdb2557-8554-4c76-bfff-2ac07023c7b8.jpg)
water of crystilisation
in the solid state water molecules that are not directly coordinated to the transition metal, which are equivalent to outer sphere ligand molecules are called ‘water crystillization’
unidentate/mono-dentate
ligands which occupy only one coordination site
ambidentate ligands
have two teeth but only one can bond at a time due to their angles
more than one potential donor atoms that could coordinate
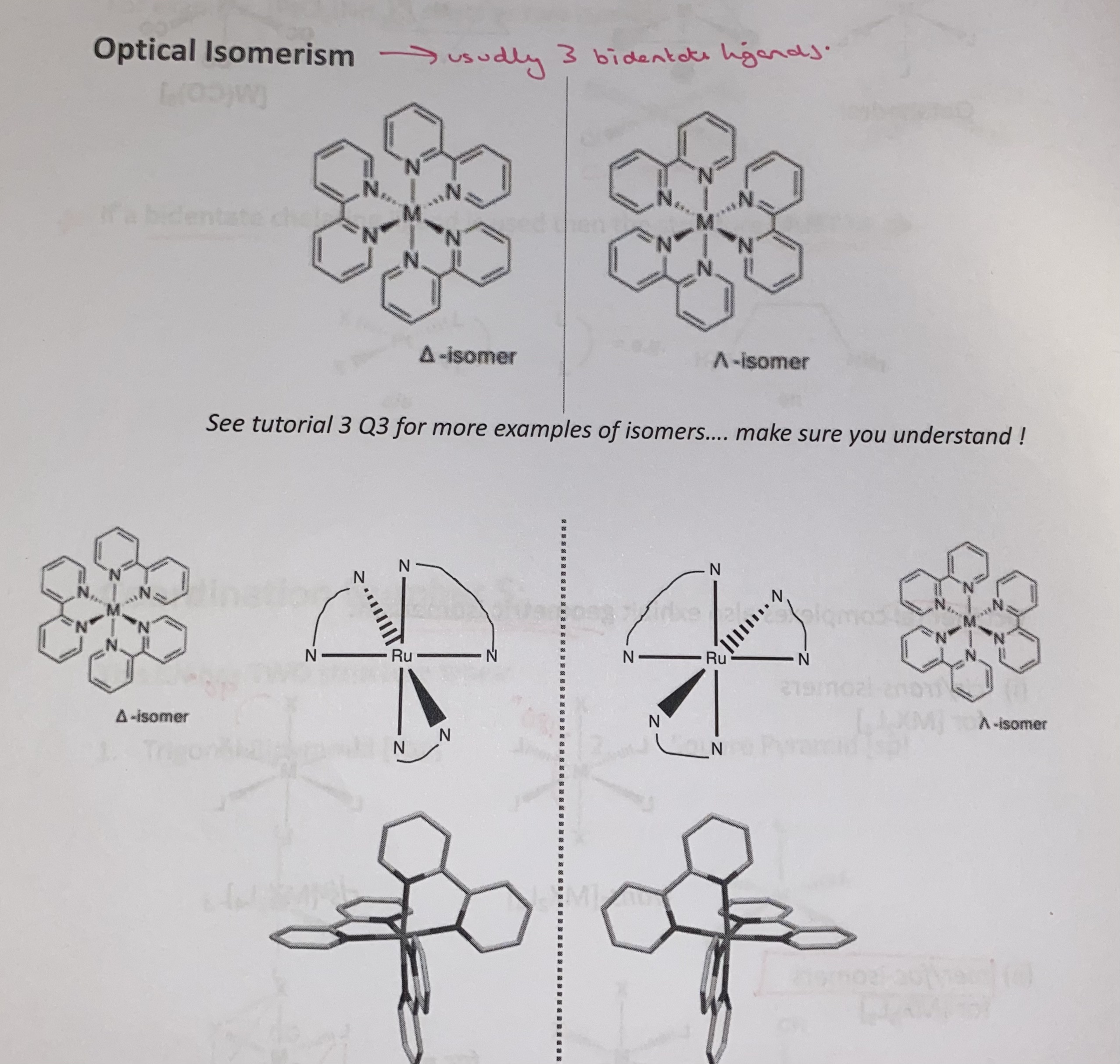
bidentate ligands
ligands with two donor atoms that are able to bond to the same metal at the same time - tehy occupy two coordination sites
tridentate ligands
ligands possessing three donor sites - occupy three coordination sites
possible geometries for complexes with coordination number of 4
tetrahedral - majority adopt this structure
square planar - seen for d^8 transition metal centres eg. some Ni2+, Pd2+, Au3+, Rh+
geometric isomers in square planar complexes
cis - like ligands are 90 degrees from each other
trans - like ligands are 180 degrees from each other
if bidentate ligand is used the the structure must be cis
possible structures of coordination number 5
trigonal bi pyramid
square pyramid
geometric isomers of octahedral complexes
cis/trans
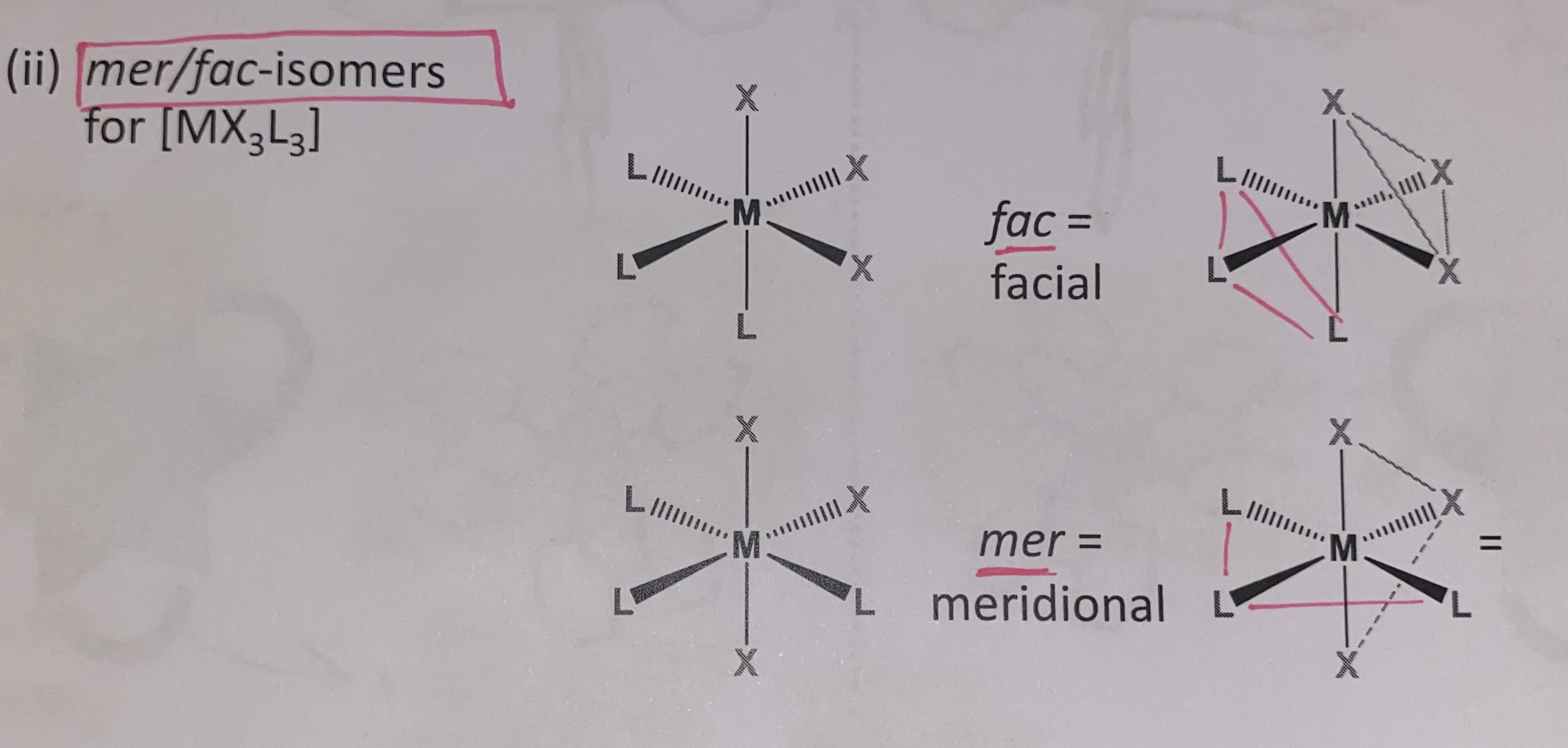
mer/fac
cis/trans geometric isomerism in octahedral complexes
trans - like ligands are 180 degrees apart
cis - like ligands are 90 degreee apart
mer/fac geometric isomerism in octahedral complexes
linkage isomerism
with ambidentate ligands
two different sites on the ligand that can each form a covalent bond
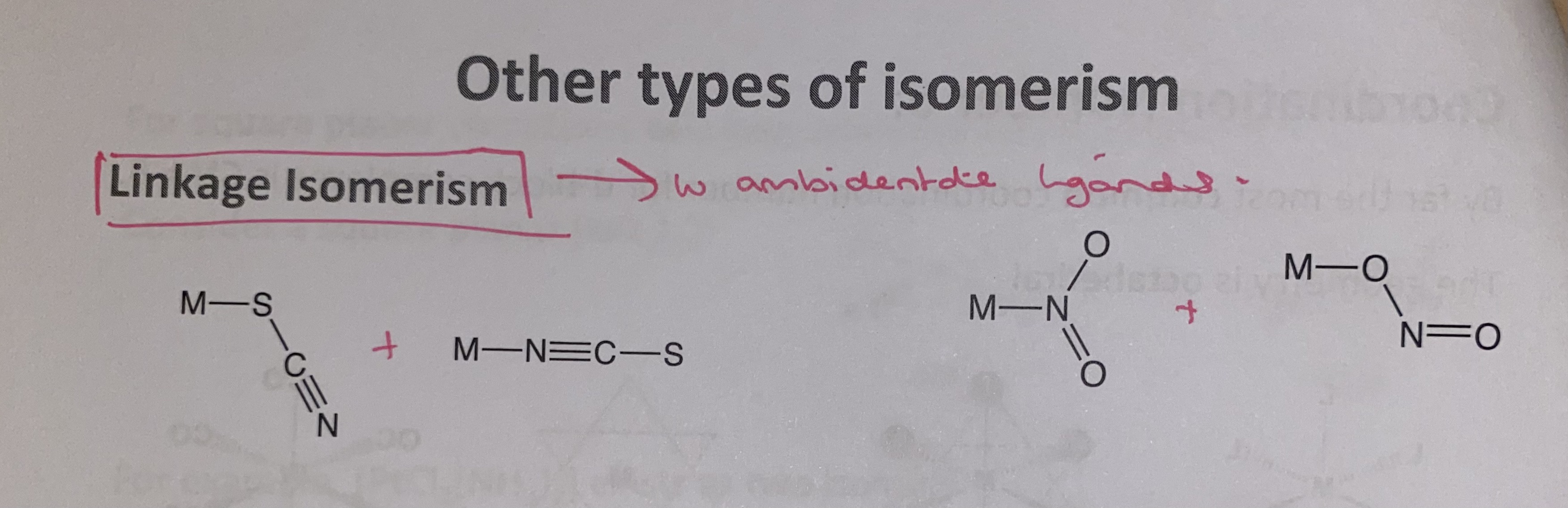
optical isomerism in octahedral complexes