Organic Chemistry 1-4
1/145
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
146 Terms
steps of naming convention
do examples
Step 1: Find the parent chain, the longest carbon chain that
contains the highest-priority functional group.
Step 2: Number the chain in such a way that the highest-priority functional group receives the lowest possible number.
Step 3: Name the substituents with a prefix. Multiples of the same type receive (di-, tri-, tetra-, etc.).
Step 4: Assign a number to each substituent depending on the carbon to which it is bonded.
Step 5: Alphabetize substituents and separate numbers from each other by commas and from words by hyphens.
Names always begin with the names of the substituents in alphabetical order, with each substituent preceded by its number. Note, however, that prefixes like di–, tri–, and tetra– as well as the hyphenated prefixes like n– and tert– (or t–) are ignored while alphabetizing. Nonhyphenated roots that are part of the name, however, are included; these are modifiers like iso–, neo–, or cyclo–.
naming of prefixes
name like alkanes but replace “ane” with “yl”
meth, eth, prop, but, pent, hex, hept, oct, non, dec
common alternative alkyl substituents, draw and identify:
sec butyl
iso butyl
isopropyl
what are alkanes
Alkanes are saturated hydrocarbons containing only single bonds between carbon atoms.
what is the formula for alkanes
The general formula for alkanes is CnH2n+2, where n is the number of carbon atoms.
draw and name alkanes from 1 to 10 carbons
what are alkenes and alkynes
Alkenes are unsaturated hydrocarbons containing at least one double bond between carbon atoms, while alkynes contain at least one triple bond.
how to name alkenes and alkynes
The double or triple bond is named like a substituent and is indicated by the lower- numbered carbon involved in the bond.
The number may precede the molecule name, as in 2-butene, or it may be placed near the suffix, as in but-2-ene; both are correct. If there are multiple multiple bonds, the numbering is generally separated from the suffix, as in 1,3-butadiene.
what is an alcohol
An alcohol is an organic compound characterized by the presence of one or more hydroxyl (-OH) groups attached to a carbon atom.
how to name an alcohol
Alcohols are named by replacing –e in the name of the corresponding alkane with –ol.
The naming rule for alcohols states that the longest carbon chain with the –OH group is identified, and the suffix –ol is added, with the position of the hydroxyl group indicated by a number.
naming rule in relation to alcohols
The chain is numbered so that the carbon attached to the hydroxyl group (–OH) gets the lowest possible number—even when there is a multiple bond present. The hydroxyl group takes precedence over multiple bonds because of the higher oxidation state of the carbon.
alcohol common names and strucutures: ethanol and 2 propanol
Examples include ethyl alcohol (rather than ethanol) and isopropyl alcohol (rather than 2-propanol.)
what are diols/glycols
Diols are organic compounds that contain two hydroxyl (–OH) groups. They can be named by identifying the carbon chain and using the suffix –diol to indicate the presence of two hydroxyl groups.
how to name diols
Diols are named by identifying the longest carbon chain, numbering the chain to give the hydroxyl groups the lowest possible numbers, and using the suffix –diol.
diols with hydroxy on the same carbon
are called geminal diols
diols with hydroxy on different carbons
are known as vicinal diols like in the same vicinity
what is an aldehyde
An aldehyde is a type of organic compound characterized by the presence of a carbonyl group (C=O) bonded to at least one hydrogen atom. It has the general formula RCHO, where R represents a hydrocarbon group.
common aldehydes names and strucutures
methanal, ethanal,
Methanal, ethanal, and propanal are referred to almost exclusively by their common names, formaldehyde, acetaldehyde, and propionaldehyde, rather than their IUPAC names.
how to name an aldehyde
To name an aldehyde, identify the longest carbon chain containing the carbonyl group, and replace the 'e' of the corresponding alkane with 'al'. Number the chain to give the carbonyl carbon the lowest possible number.
what are ketones
Ketones are organic compounds characterized by a carbonyl group (C=O) bonded to two carbon atoms. They have the general formula RCOR', where R and R' represent hydrocarbon groups.
how to name ketones
To name a ketone, identify the longest carbon chain containing the carbonyl group, replace the 'e' of the corresponding alkane with 'one', and number the chain to give the carbonyl carbon the lowest number possible.
common ketones and strucutres
acetone
carboxylic acid structure and common names and naming scheme
methanoic acid
ethanoic acid
formic acid= methanoic acid
acetic acid= ethanoic acid
notice naming scheme: form means 1 and acet means 2
ester structure and how to name
oate
amide structure and how to name
anhydride structure and how to name
amide structure
types of amides, amines and alcohols and draw them out
primary secondary and tertiary
ether structure
prefixes of functional groups that are not the priority
alcohol
aldehyde
ketone
ester
amide
hydroxy
oxo
keto
alkoxycarbonyl
carbamoyl/amide/amido
order of priority for functional groups
what are the four quantumn numbers
n, l, ml, ms
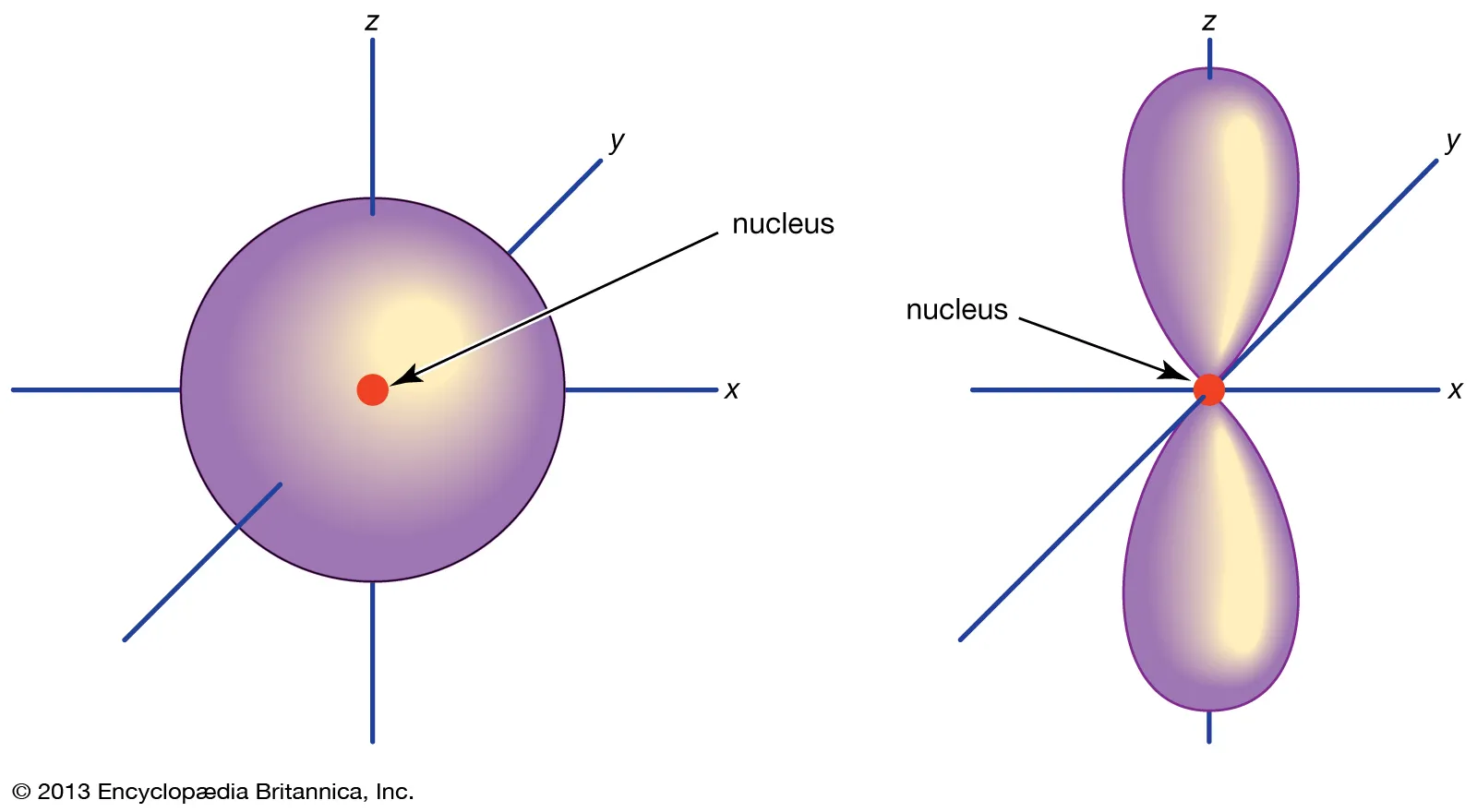
shape of an s orbital in relation to the nucleus
An s-orbital is spherical and symmetrical, centered around the nucleus.
shape of the p orbital in relation to the nucleus
p-orbital is composed of two lobes located symmetrically about the nucleus
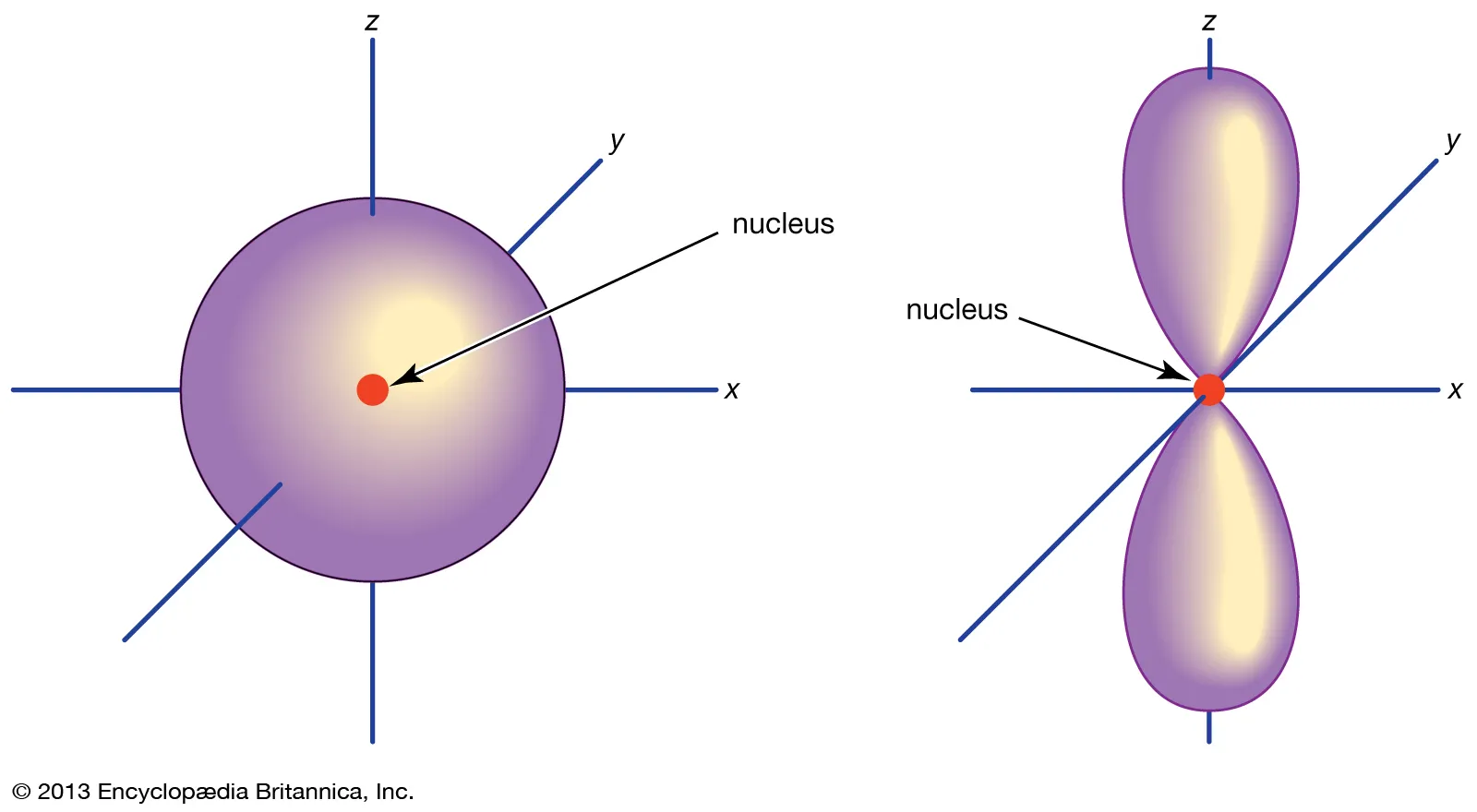
types of p orbitals
Picture the p-orbital as a dumbbell that can be positioned in three different orientations, along the x-, y-, or z-axis. It should make sense that there are three p-orbitals;
what orbitals are formed and how
molecular orbitals are formed from the combination of atomic orbitals
how are molecular orbitals formed
Molecular orbitals are obtained mathematically by adding or subtracting the wave functions of the atomic orbitals.
if the signs of the wave functions are the same
the atomic orbitals combine constructively, resulting in a bonding molecular orbital
if the signs of the wave functions are different
the atomic orbitals combine destructively, resulting in an antibonding molecular orbital.
what is true about the energy of bonding and antibonding orbitals
Bonding orbitals have lower energy than the original atomic orbitals
antibonding orbitals have higher energy than the original atomic orbitals
making them less stable.
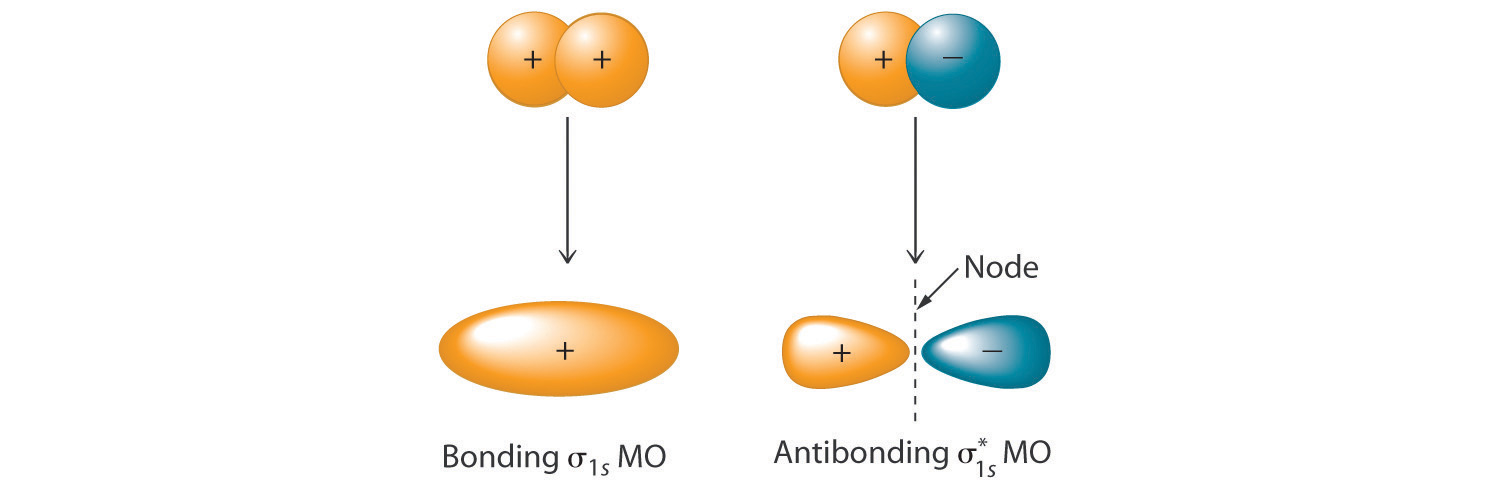
what is a sigma bond
When a molecular orbital is formed by head-to-head or tail-to-tail overlap,
what is a pi bond
When two p-orbitals line up in a parallel (side-by-side) fashion, their electron clouds overlap, and a bonding molecular orbital, called a pi (π)bond, is formed.
what to note about multiple bonds
it is possible to break only one of the bonds in a double bond, leaving a single bond intact. This happens often in organic chemistry, such as when cis–trans isomers are interconverted between conformations.
is it harder to break sigma or pi bond
A pi bond is generally weaker than a sigma bond due to its formation from lateral overlap of p-orbitals, making it easier to break compared to a sigma bond. but easier to break single bond than multiple bond because it includes both types of bonds
what is true about single, double and triple bonds in relation to rotation
As discussed previously, double and triple bonds do not freely rotate like single bonds. As such, double bonds in compounds make for stiffer molecules. Partial double-bond character in structures with resonance also restricts free rotation, resulting in more rigid structures. Proteins exhibit this kind of limited rotation because there is resonance in the amide linkages between adjacent amino acids.
what is hybridization
Hybridization is the mixing of atomic orbitals to create new hybrid orbitals that can form sigma bonds or accommodate lone pairs in a molecule, influencing molecular geometry and bonding properties.
how are sp3 hybrid orbitals formed
Sp3 hybrid orbitals are formed by the mixing of one s orbital and three p orbitals from the same atom, resulting in four equivalent orbitals that are arranged in a tetrahedral geometry with 109.5 bond angles
percentage s character and p character in sp3 hybrid orbitals
Sp3 hybrid orbitals consist of 25% s character and 75% p character, reflecting the contribution of one s orbital and three p orbitals in their formation.
how does sp2 hybridization occur
Sp2 hybridization occurs when one s orbital and two p orbitals of an atom mix to form three equivalent sp2 hybrid orbitals, which are arranged in a trigonal planar geometry with 120-degree bond angles between the orbitals
what to note about the s and p orbitals in relation to hybridization and alkenes
This is the hybridization seen in alkenes. The third p-orbital of each carbon is left unhybridized.
This is the orbital that participates in the π bond. The three sp
2 orbitals are oriented 120° apart, which allows for maximum
separation.
We know that the unhybridized p-orbital is involved in the π
component of the double bond, but what about the hybrid orbitals?
In ethene, two of the sp2 hybridized orbitals will participate in C–H bonds, and the other hybrid orbital will line up with the π bond and form the σ component of the C=C double bond.
percentage s and p character in sp2 orbitals
Sp2 hybrid orbitals consist of 33% s character and 67% p character, indicating the contribution of one s orbital and two p orbitals in their formation.
how are sp hybrid orbitals formed
Sp hybrid orbitals are formed when one s orbital and one p orbital mix to produce two equivalent sp hybrid orbitals. These orbitals are oriented 180° apart, resulting in a linear geometry.
what is true about the s and p orbitals in sp hybridization in relation to alkynes
To form a triple bond, we need two of the p-orbitals to form π bonds, and the third p-orbital will combine with the s-orbital to form two sp-orbitals, as shown in Figure 3.7.
These orbitals have 50% s character and 50% p character. These orbitals are oriented 180° apart, which explains the linear structure of molecules containing sp-hybridized carbons.
The two π bonds can be between the carbon and one other atom (forming a triple bond, like ethyne), or between the carbon and two different atoms (forming two double bonds in a row, like carbon dioxide). In both cases, the molecule is linear about the sp-hybridized carbon.
what is resonance delocalization
can hybridized orbitals form pI bonds
Resonance delocalization of electrons occurs in molecules that have conjugated bonds.
Conjugation requires alternating single and multiple bonds because this pattern aligns a number of unhybridized p-orbitals down the backbone of the molecule.
π electrons can then delocalize through this p-orbital system, adding stability to the molecule.
No, hybrid orbitals typically don't form pi bonds; rather, pi bonds are formed by the side-by-side overlap of unhybridized p orbitals, while hybrid orbitals are involved in sigma bonding.
what does an acid become
conjugate acid
what does a base become
conjugate base
when will the acid base reaction proceed
when the products are weaker than the reactants
what is a lewis acid
a substance that can accept an electron pair.
what is a lewis base
a substance that can donate an electron pair.
bronsted Lowry acid
a substance that donates a proton.
bronsted Lowry base
a substance that accepts a proton.
what is an amphoteric substance and example
An amphoteric substance is one that can act as both an acid and a base, depending on the reaction environment. A common example is water, which can donate a proton to become hydroxide or accept a proton to become hydronium.
what is Ka
The acid dissociation constant, a measure of the strength of an acid in solution.
ka formula given acid HA
is Ka = [H+][A-]/[HA].
how to calculate pka
The pKa can be calculated using the formula pKa = -log(Ka), where Ka is the acid dissociation constant.
what is pka
The negative logarithm of the acid dissociation constant (pKa = -log Ka), indicating the strength of an acid; lower pKa values represent stronger acids.
what does low vs high Pka indicate
Low pKa indicates a stronger acid, while high pKa suggests a weaker acid. A lower pKa value means the acid dissociates more completely in solution.
pka values for weak acid
-2 to 20
periodic table trend for acidity
Generally, bond strength decreases down the periodic table, and acidity therefore increases.
Also, the more electronegative an atom, the higher the acidity.
When these two trends oppose each other, low bond strength takes precedence.
so acidity increases across a period and down a group
what to note about certain hydrogens
For the common functional groups on the MCAT, the α-hydrogens of carbonyl compounds deserve special note.
α-hydrogens are connected to the α-carbon, which is a carbon adjacent to the carbonyl.
Because the enol form of carbonyl-containing carbanions is stabilized by resonance, these are acidic hydrogens that are easily lost.
functional groups that act as acids
aldehydes, ketones, alcohols, carboxylic acid and carboxylic acid derivtatives
functional groups that act as bases
amines and amides
what is a nucleophile
A nucleophile is a chemical species that donates an electron pair to form a chemical bond in a reaction.
Nucleophiles are typically characterized by having a negative charge or an atom with a lone pair of electrons or pi bonds (double, triple bonds)
what is true about nucleophiles and bases
good nucleophiles tend to be good bases. there is a difference, however.
Nucleophile strength is based on relative rates of reaction with a common electrophile—and is therefore a kinetic property.
Base strength is related to the equilibrium position of a reaction—and is therefore a thermodynamic property.
factors affecting nucleophilicity
Charge: Nucleophilicity increases with increasing electron density (more negative charge)
Electronegativity: Nucleophilicity decreases as electronegativity increases because these atoms are less likely to share electron density
Steric hindrance: Bulkier molecules are less nucleophilic
Solvent: Protic solvents can hinder nucleophilicity by protonating the nucleophile or through hydrogen bonding
how does solvent affect nucleophilicity and memory device
In polar protic solvents, nucleophilicity increases down the periodic table. In polar aprotic solvents, nucleophilicity increases up the periodic table.
PP DOWN
what is a polar protic solvent
A solvent that can form hydrogen bonds and has hydrogen atoms bonded to electronegative atoms like oxygen or nitrogen, allowing it to stabilize ions through solvation. These solvents typically include water, alcohols, and carboxylic acids. (so the H needs to be attached to NOF)
can we use non polar solvents in nucleophiles electrophile reactions
We can’t use nonpolar solvents in these nucleophile–electrophile reactions because our reactants are polar—they wouldn’t dissolve!
what are electrophiles
Electrophiles are species that accept electrons in a chemical reaction, often containing a positive charge or a partial positive charge. They react with nucleophiles, which are electron-rich species.
what is true about acids and electrophiles
Again, this definition brings to mind Lewis acids. The distinction, as with nucleophiles and bases above, is that electrophilicity is a kinetic property, whereas acidity is a thermodynamic property. Practically, however, electrophiles will almost always act as Lewis acids in reactions.
what factors make an electrophile stronger
Factors that make an electrophile stronger include a greater positive charge, a better leaving group, and the ability to stabilize the resulting negative charge after the reaction. If empty orbitals are present, an incoming nucleophile can make a bond with the electrophile without displacing the leaving group.
what are some common electrophiles
Electrophilicity and acidity are effectively identical properties when it comes to reactivity. Just as alcohols, aldehydes and ketones, carboxylic acids, and their derivatives act as acids, they also act as electrophiles, and can make good targets for nucleophilic attack.
review what makes a better vs worse electrophile, compare and do examples
what are leaving groups
Molecular fragments that retain the electrons after
heterolysis.
what makes a good leaving group
is a species that can stabilize the negative charge after detaching from a molecule, facilitating reaction progress. Common good leaving groups include halides (the conjugate bases of strong acids) and tosylates.
types of nucleophilic substitution reactions
are SN1 and SN2 reactions, which differ in mechanism and kinetics.
what is an sn1 reaction
A nucleophilic substitution reaction that occurs in two steps, involving the formation of a carbocation intermediate.
what to note about an sn1 reaction
The first step is the rate-determining step where the leaving group departs, followed by nucleophilic attack.
rate law of an sn1 reaction
Rate = k [substrate]
first order reaction
what does an SNL reaction form
Because SN1 reactions pass through a planar intermediate before the nucleophile attacks, the product will usually be a racemic mixture.
The incoming nucleophile can attack the carbocation from either side, resulting in varied stereochemistry.
what is an sn2 reaction
A bimolecular nucleophilic substitution reaction that involves a single transition state. It occurs in one concerted step, leading to stereochemical inversion of the substrate.
rate law of an sn2 reaction
Rate = k [substrate][nucleophile], second order reaction.
what to note about sn2 reactions
The nucleophile must perform a backside attack, which leads to inversion of stereochemistry. (R) and (S) is also changed if
the nucleophile and LG have the same priority level. SN2
prefers less-substituted carbons because steric hindrance
inhibits the nucleophile from accessing the electrophilic
substrate carbon.
what is oxidation reduction
A chemical reaction that involves the transfer of electrons between two substances, resulting in the oxidation of one species and the reduction of another.
what is oxidation
The process in which a substance loses electrons, increasing its oxidation state.
how to detect oxidation
view oxidation as increasing the number of bonds to oxygen or other heteroatoms (atoms besides carbon and hydrogen).
what is reduction
decrease in oxidation
how to detect reduction
reduction as increasing the number of bonds to hydrogen.
another way to view oxidation
Oxidation of a carbon atom occurs when a bond between a carbon atom and an atom that is less electronegative than carbon is replaced by a bond to an atom that is more electronegative than carbon.
In practice, this usually means decreasing the number of bonds to hydrogen and increasing the number of bonds to other carbons, nitrogen, oxygen, or halides.
what is the oxidizing agent
A substance that increases oxidation by accepting electrons or being reduced in a chemical reaction.