Chapter 18 - the cell cycle
1/97
Earn XP
Description and Tags
powerpoint and textbook notes
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
98 Terms
what is the cell cycle?
orderly sequence by which a cell duplicates its contents and divides into 2
essential mechanism that all living things reproduce
Cells reproduce by?
they duplicate their contents and then divide in two→ process called the cell cycle
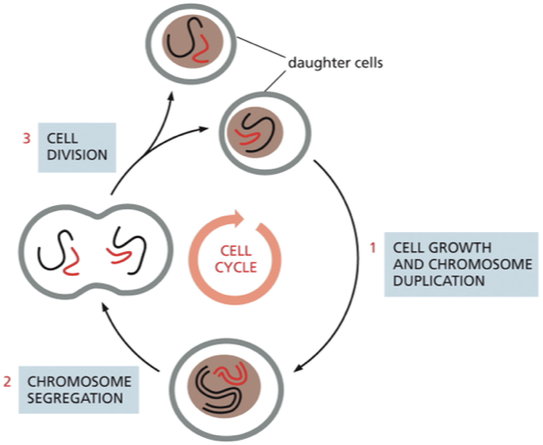
Cell cycle control system
is a control system that regulates the progression through each phase of the cell cycle. Involves the activation of specific cell division cycle proteins (Cdc)
makes sure that the events in the cell cycle happen in a set sequence and that each process has been completed before the next one begins
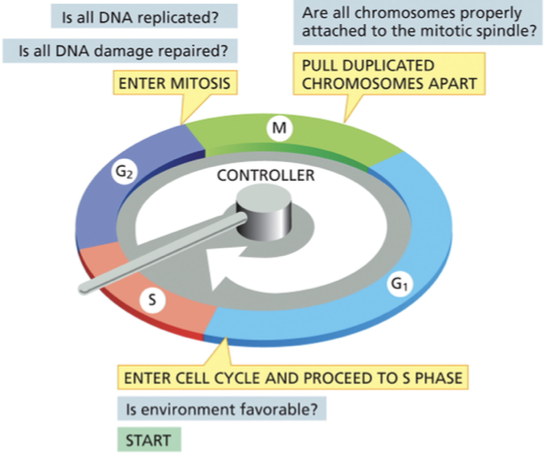
checkpoints: are transition points, progression of the cell cycle is regulated, allows or prohibits the cells progression to the next stage
What happens at each check point in the cell cycle?
cell cycle control system regulates progression through the cell cycle at 3 main transition points
G1/ S checkpoint also known as START: transition at the end of G1 phase, once you get through this transition point it commits the cell to enter the cell cycle and continue to S phase
is the environment favorable for proliferation before proceeding to replicate its DNA
if extracellular conditions are unfavorable → cells can enter G0 (resting state)
G2/M checkpoint
Is the DNA undamaged & fully replicated
Spindle assembly checkpoint
confirms that the duplicated chromosomes are properly attached to the miotic spindle before the spindle pulls the chromosomes apart and segregates them into daughter cells
What are the phases of the cell cycle?
Interphase: long period of the cell cycle between one mitosis and the next it includes G1, S phase, and G2 phase
G1 phase(gap) : protein synthesis, growth, decision about cell division
S phase: DNA is synthesized
G2 phase (gap) : protein synthesis in preparation for mitosis growth
M phase: nucleus and cytoplasm divide to produce 2 daughter cells
mitosis (nuclear division)
cytokinesis (cytoplasmic division)

the cell cycle control system governs the cell cycle by activating and deactivating key proteins and protein complexes that initiate or regulate DNA replication, mitosis, and cytokinesis. This regulation is carried out by?
phosphorylation followed by dephosphorylation of proteins (most common way to switch activity of a protein on and off)
phosphorylation= protein kinases
dephosphorylation= protein phosphatases
cyclins and cyclin dependent protein kinases (Cdk)
Cdk binds to cyclin (protein)→ enzymatically active
Once activated, cyclin–Cdk complexes help trigger various cell-cycle events, such as entry into S phase or M phase
cyclin: no enzymatic activity by themselves
concentration of cyclin vary in a cyclical fashion during the cell cycle
cyclin-dependent kinases (Cdk) - kinases that are always present in the cell but not always active
require interaction with cyclin to become active
Different cyclin-Cdk complexes trigger different steps in the cell cycle:
what are the 3 major classes of cyclin- cdk complexes
when do they rise and fall
G1/ S Cyclins → bind to Cdk proteins→ forms G1/S-Cdk and S-Cdk
G1/S-Cdk: initiates the cell cycle
S- cyclin
S-Cdk: starts the S phase
M-cyclin
M-cdk - triggers the events of M phase
•different cyclins are produced and degraded at different phases of the cell division cycle
G1/ S rises @ beginning of G1 and falls before S phase
S cyclin rises before the start of S phase and falls during M phase
M cyclin starts rising during G2 , peaks when mitosis starts and falls before M phase ends.

How does the concentration of M-cyclin relate to the M-cdk activity
Inc conc of M-cyclin during mitosis → inc in formation of M-cdk (drives entry into the M phase)
what are the events that are triggered by M-Cdk?
•chromatin condensation: phosphorylation of condensins
•nuclear envelope breakdown: phosphorylation of lamins and nuclear pore complexes
•fragmentation of the Golgi apparatus: phosphorylation of Golgi matrix proteins
•formation of the mitotic spindle: phosphorylation of centrosome and microtubule-associated proteins
M-Cdk is also known as ________
maturation promoting factor(MPF)
How was MPF activity discovered?
Figure 18–7 MPF activity was discovered by injecting Xenopus egg cytoplasm into Xenopus oocytes. (A) A Xenopus oocyte is injected with cytoplasm taken from a Xenopus egg in M phase. The cell extract drives the oocyte into M phase of the first meiotic division (a process called maturation), causing the large nucleus to break down and a spindle to form. (B) When the cytoplasm is instead taken from a cleaving egg in interphase, it does not cause the oocyte to enter M phase. Thus, the extract in (A) must contain some activity—a maturation-promoting factor (MPF)—that triggers entry into M phase.
from the powerpoint notes
Frog oocytes are arrested at the G2 phase of meiosis I
•a G2-arrested oocyte can be driven to enter M-phase by injecting cytoplasm from an M-phase donor cell
•a G2-arrested oocyte injected with cytoplasm from an interphase donor cell, will not enter M-phase

the concentration of a given protein in the cell is determined by the rate at which the protein is ________ and the rate that it is _______
synthesized
degraded
The gradual increase in cyclin concentration stems from _____________ of cyclin genes and synthesis of cyclin proteins, where as the rapid fall in cyclin concentration is caused by ?
transcription
caused by the targeted destruction of the protein
the degradation of M- and S- cyclins midway through M phase depends on a enzyme called?
anaphase - promoting complex or cyclosome (APC/C)
tags cyclins w/ a chain of ubiquitin→ directed to proteasomes→ rapidly degraded → returns Cdk to an inactive state
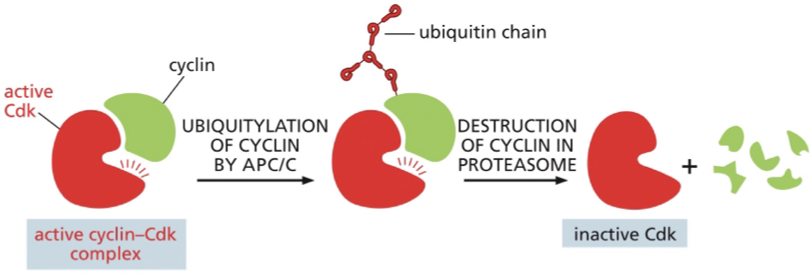
Each cyclin is inactivated by
the selective degradation of its associated cyclin
the destruction of M-cyclin occurs?
•near the end of M-phase and follows the ubiquitin pathway
During mitosis anaphase promoting complex (APC) adds?
ubiquitin to cyclin and to other proteins involved in the regulation of mitosis
M-cyclin degradation—and the resulting inactivation of M-Cdk—leads to the molecular events that
take the cell out of mitosis.
although cyclin concentrations increase gradually, the activity of the associated cyclin-cdk complexes switch on abruptly at the appropriate time in the cell cycle. What triggers the abrupt activation of these complexes?
cyclin-Cdk complexes have inhibitory phosphates
Cdk become activated by dephosphorylation by a specific protein phosphatase
For M-cdk to be active…
inhibitory phosphatases must be removed
process
M-cdk complex is formed→ phosphorylated at adjacent sites by protein kinase Wee1→ conformation keeps M-Cdk in an inactive state until the phosphates are removed by activating protein phosphatase called Cdc25
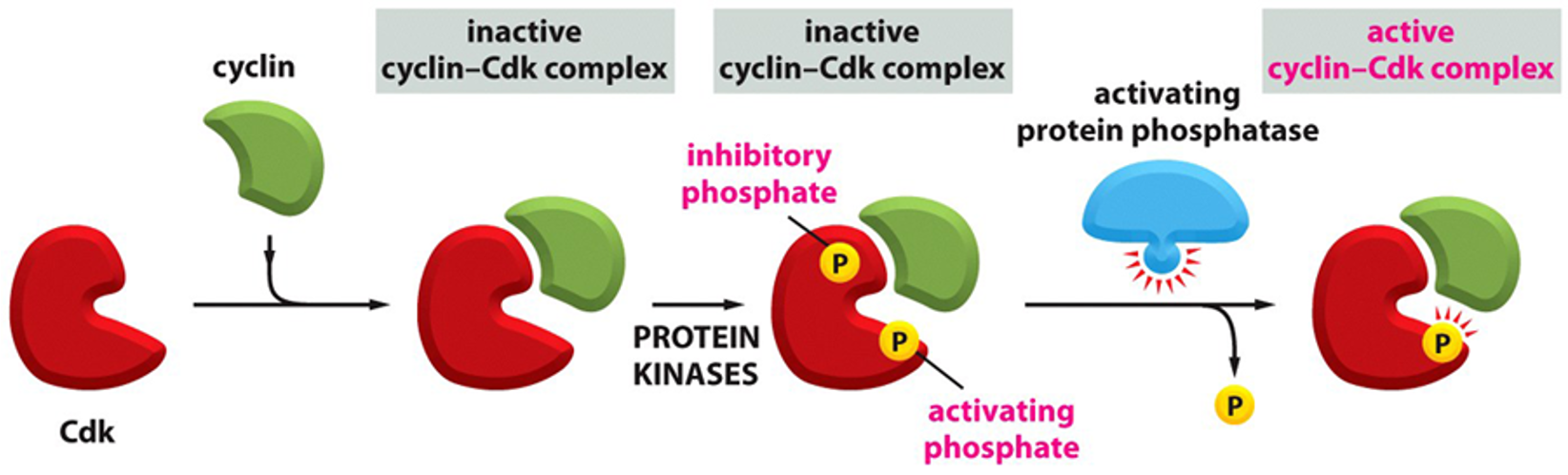
What would be the result of an overactive wee-1 kinase?
M-cdk would never become active and the cell would never enter mitosis
CDKs are also regulated via phosphorylation
•M-cdk is phosphorylated with an inhibitory P via the _____
•M-cdk is phosphorylated with an activating P via _____
•The inhibitory phosphate is removed by the phosphatase Cdc25
Wee1 kinase
CAK
In addition to phosphorylation and dephosphorylation the activity of Cdks can be regulated by binding of?
Cdk inhibitors
blocks assembly or activity of certain cyclin Cdk complexes @ certain times
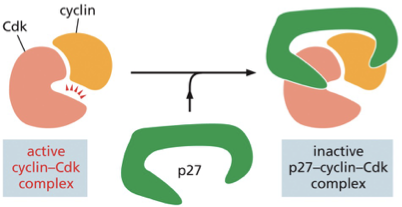
In regards to inhibitor proteins what does p27 do?
The p27 protein regulates G1 cyclin and prevents progression into S phase
process:
active cyclin-Cdk inhibitor→ p27 binds to the complex→ inactive p27-cyclin-cdk complex (prevents the Cdk from phosphorylation target proteins required for progress through G1 into S phase)
side note:
pausing at this transition point in G1 gives the cell more time to grow or allows it to wait until extracellular conditions are favorable for division
Phosphorylation and dephosphorylation do more than just regulate the activation of Cdks
________ also reverse the downstream effects of Cdks by?
Protein phosphatases→ remove the phosphates that Cdks add to their target protein
PP2A & PP2A-B55
PP2A
family of phosphatases
regulator that opposes Cdk
enzyme— consist of 3 subunits
PP2A-B55
targets proteins phosphorylated by M-Cdk
Early in mitosis when the activity of M- Cdk rises, M-Cdk shuts off activity of PP2A-B55→ result= M-Cdk (early in mitosis) operates unopposed→leads to rapid phosphorylation of target proteins
When M-Cdk is inactivated (by degredation of M-cyclin by APC/C) the system reverses direction → PP2A-B55 becomes reactivated and dephosphorylates M-Cdks targets
form of positive feedback

What is M-Cdk?
the cyclin-Cdk complex that drives entry into mitosis
Most cyclins with result in both positive and negative regulation of their own activity—ultimately resulting in ?
the degradation of their cyclin
regarding the cell cycle control system and the check points fill in the blanks
allows entry into the cell cycle and initiation of S phase only if ________
triggers mitosis only after____________
initiates chromosome segregation after ____________
environmental conditions are appropriate
the DNA has been completely replicated
the duplicated chromosomes are correctly aligned on the mitotic spindle
Control system & Cdks
at the start check point in late G1 phase…
at the G2 -to-M transition its supreses?
can delay chromosome segregation in mitosis by inhibiting?
Cdk inhibitors to keep cells from entering the cell cycle
suppresses the activation of M-Cdk by inhibiting the phosphatase required to activate Cdk
inhibiting the activation of APC/C → prevents the degradation of M-cyclin

Tight controls of the activities of cyclin/cdk complexes ensure?
that the cell proceeds through the cell cycle only when ready
checkpoints: the cell cycle is halted at these points if?
a problem is detected
the cell cycle continues after the problem is fixed
checkpoints also ensure that cell division cycle events occur in the proper sequence
How do cyclin–Cdk complexes trigger different stages of the cell cycle?
The kinase activity of the Cdk subunit increases at different stages of the cycle.
Cdk protein levels are constant in the cell cycle, but they are activated by interacting with a series of cyclin proteins that rise and fall at specific cell-cycle stages. Cyclin degradation causes inactivation of the Cdk.
The cell-cycle control system initiates chromosome segregation only after which of the following has occurred?
The duplicated chromosomes are correctly aligned on the mitotic spindle.
What are the 3 diferent paths a cell could take at the Start checkpoint?
G0- non proliferative state, if appropriate signals are given can go back into the cell cycle
Terminal differentiation
cell changes its physiology becomes a defined cell
should stay out of the cell cycle - shuts down its checkpoints→ when they acquire mutations that let them go into the cell cycle it is extremely oncogenic bc they dont have any checkpoints (no regulation at the checkpoints)
overactive cdks can cause this to slip back into the cell cycle
S phase
discuss how and why Cdks are inactivated in early G1`
Cdks Inactivated in Early G₁ — Key Points
• At the end of M phase, all S-Cdks and M-Cdks must be shut down so the cell finishes division and doesn’t start another round immediately.
• Transition from M → G₁ requires complete inactivation of Cdk activity.
• The cell does this by:
• Destroying existing cyclins
• Blocking new cyclin synthesis
• Using Cdk inhibitor proteins (CKIs) to suppress any remaining cyclin-Cdk complexes
• Using multiple mechanisms ensures robust shutdown of Cdks.
• This reset creates a stable early G₁ phase, allowing the cell to grow and check its environment before starting a new cycle.
mammalian cells will multiply only if they are stimulated to do so by extracellular signals called?
Mitogens
extracellular signal produced by other cells
if the cell doesnt get these signals it arrests in G1, if the cell is deprived from mitogens for long enough than it will go back into G0
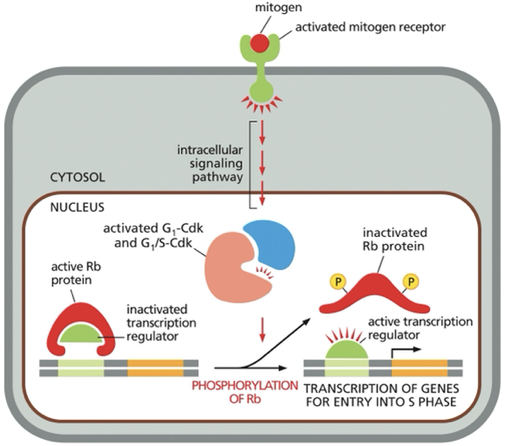
Mitogens signals ultimately lead to?
activation of the G1 and G1/S Cdks
•These kinases result in hyper-phosphorylation of the Retinoblastoma (Rb) protein
•This results in de-activation of Rb and removal of its repression upon transcription factors
•TFs are now active to turn on genes necessary for entrance into S-phase
retinoblastoma (Rb) protein
crucial negative control
initially identifies from studies on rare child hood tumor called retinoblastoma
abundant in the nuclei of all vertebrate cells→ binds to transcription regulators → prevents them from turning on genes required for cell proliferation
One way that mitogens stimulate cell proliferation is by inhibiting?
what is the process?
retinoblastoma (Rb) proteins
2 process:
activated Rb protein binds to transcription regulator → inactivates transcription regulator (no transcription of genes goin on)
Mitogen (signal) → binds to cell surface receptor→ activated mitogen receptor → activate intracellular signaling → lead to formation/ activation of G1-Cdk and G1/S-Cdk complexes→ complexes phosphorylate Rb this inactives Rb→ Rb releases its transcription regulators (needed to activate the transcription of genes required for entry into S phase
in the absence of mitogens, dephosphorylated Rb proteins?
hold specific transcription factors in an inactive state

A
Once through the START checkpoint the cell will no longer respond to extracellular signal but will respond to ?
internal signals
DNA damage will trigger a signaling pathway to?
HALT the cell cycle
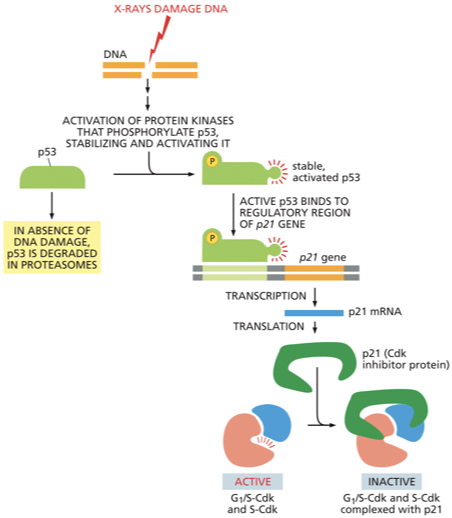
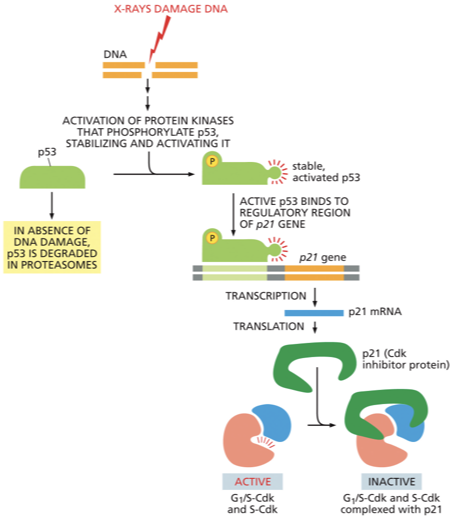
p53
DNA damage in G1 causes an increase in both the concentration and activity of this protein
p53 is a transcription regulator that triggers the production of p21- a Cdk inhibitor
p21 protein binds to G1/ S-Cdk and S-Cdk preventing the cell from going into S phase → cell arrests in G1
p21 inhibits cyclin-cdk complexes
If damage is too severe to be repaired p53 will?
induce apoptosis (programmed cell death)
If active long enough p53 will?
trigger apoptosis
If p53 is missing or defective what can happen?
the unrestrained replication of damaged DNA can lead to a high rate of mutation →lead to cancer
When DNA is damaged, specific protein kinases respond by both activating the
p53 protein and halting its otherwise rapid degradation
most liver cells are in what phase of the cell cycle
G0 - but they can be stimulated to proliferate if the liver is damaged
Most of the diversity in rates of cell division in the adult body is due to variations in the length of which cell-cycle phase(s)?
G1 phase and G0 phase
The initiation of DNA replication takes place in two steps. describe what happens?
DNA replication is triggered by S-Cdk activity
During G1
Cdc6, ORC and helicases bind to origins of replication
Active S-Cdk phosphorylates all three:
tagging Cdc-6 for degradation- by phosphorylating cdc-6 it blocks re-replication → preventing the initiation of DNA replication, wont recruit helicase to location
inactivating ORC- blocks re-replication - cant bind another cdc 6
Activates the helicases by phosphorylation this allows them to interact with DNA polymerase

When is cdc-6 produced
after mitosis and early in G1
S-cdk triggers production of M cyclins and activation of
Mcdk
M-Cdk
helps prepare unduplicated chromosomes for segregation
induces assembly of the mitotic spindle (pulls the duplicated chromosomes apart)
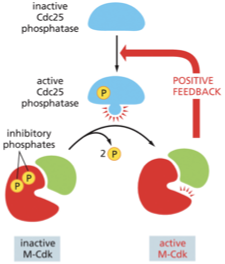
accumulate throughout G2, activated at the end of G2 when the activating phosphatase Cdc25 removes the inhibitory phosphates that were keeping M-Cdk inactive
activated M-cdk indirectly activates more M-Cdk creating a positive feedback loop
Activated M-cdk shuts down the inhibitory kinase Wee1→ promotes the production of activated M-cdk
once M-Cdk activation begins, it ignites an explosive increase in M-Cdk activity that drives the cell abruptly—and irreversibly—from G2 into M phase.
Activation of Mcdk triggers condensation of ?
chromosomes
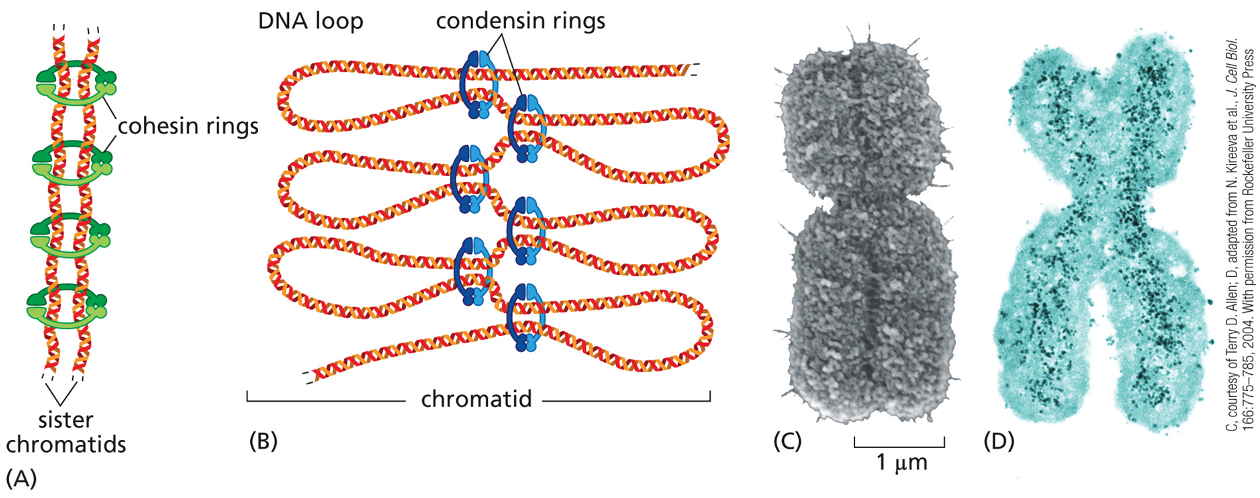
When a chromosome is duplicated, the 2 copies remain tightly bound together, these sister chromatids (identical copies) are held together by protein complexes called?
cohesins
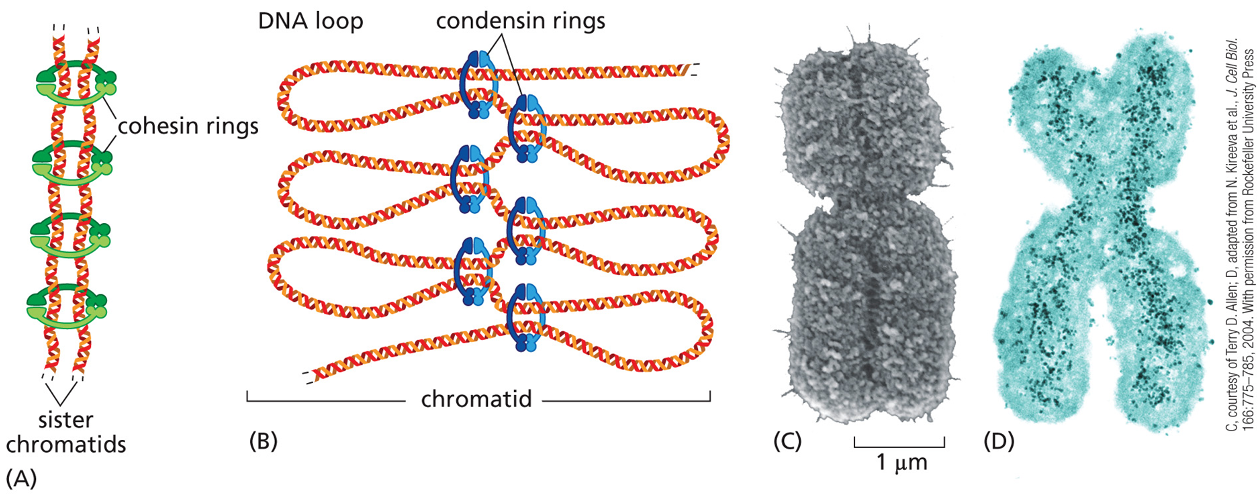
the cohesion between sister chromatids is crucial for?
proper chromosome segregation
defects can lead to major errors in chromosome segregation — in humans can lead to abnormal # of chromosomes→ aneuploidy
chromosome condensation
duplicated chromosome becomes packed in a more compact structure prior to cell division
chromosome condensation is carried out early in mitosis, by protein complexes called?
condensins
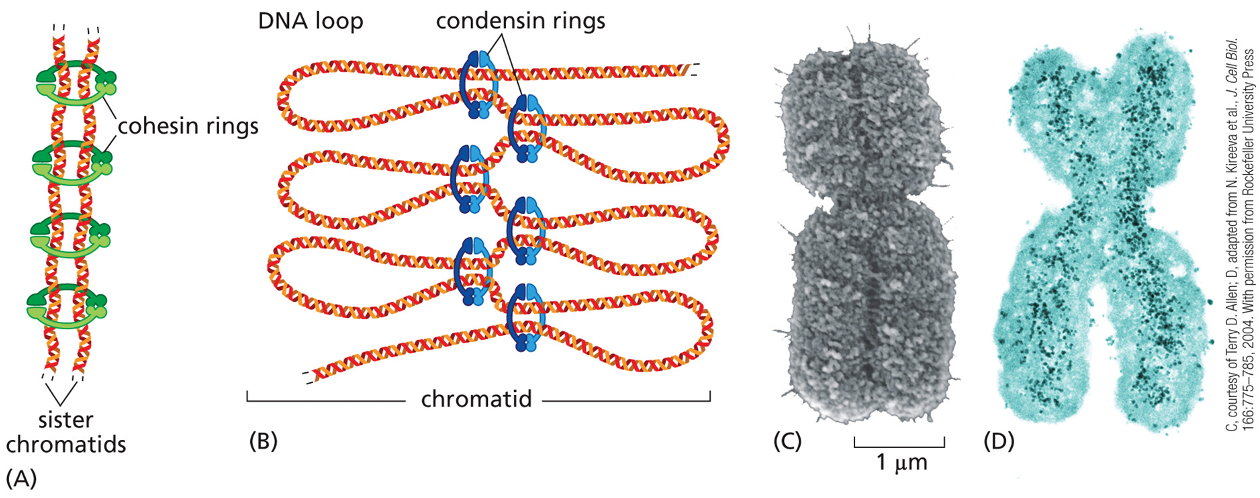
cohesins & condensins are structurally related because both?
form ring structures around chromosomal DNA
however cohesins make a ring around the two sister chromatids tying them together, while the condensins assemble along each individual sister chromatid helping making them a more compact
What does the phosphorylation of Cdc25 by M-Cdk do?
It activates Cdc25, which in turn activates more M-Cdk.

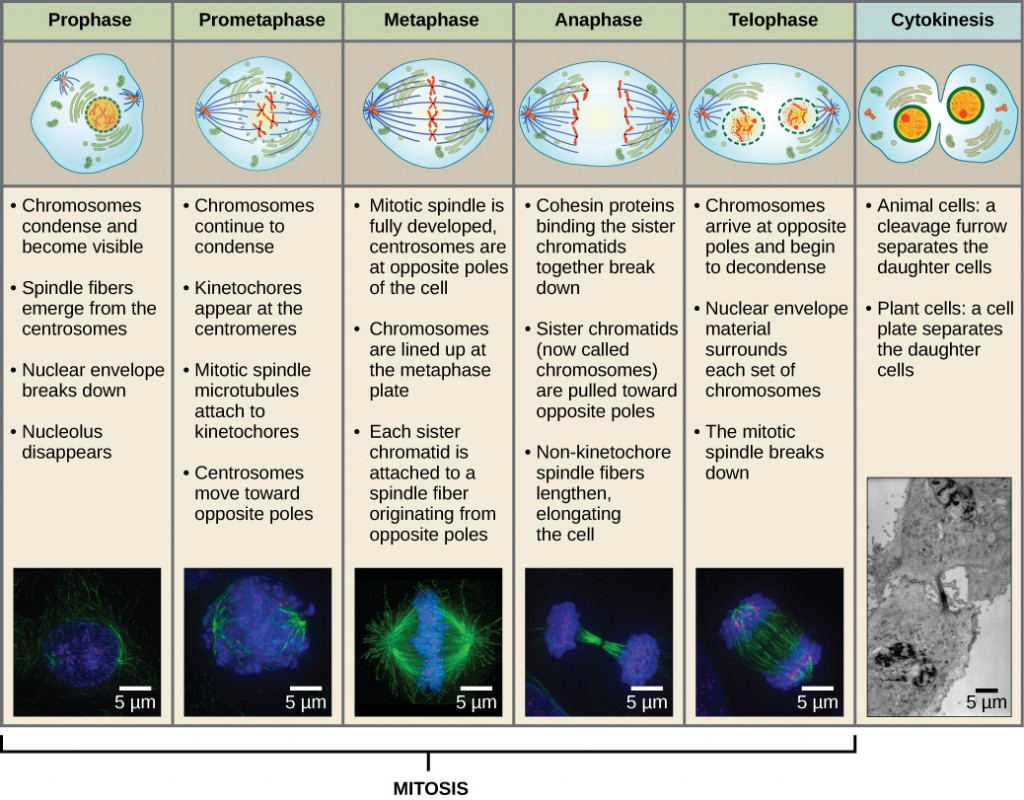
The chromosomes attach to the mitotic spindle at what stage of the cell cycle
prometaphase
the assembly of the mitotic spindle begins in ______
prophase
but the spindle microtubules cannot make contact with chromosomes untul the nuclear envelope breaks down
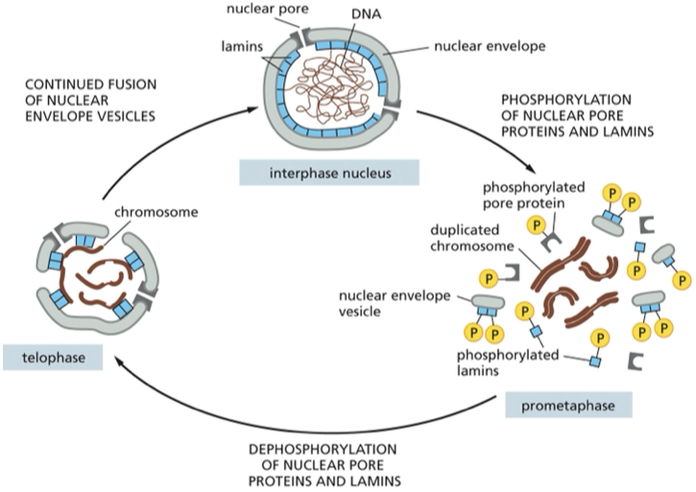
When does the nuclear envelope break down?
prometaphase
the process is triggered by the phosphorylation + disassembly of nuclear pore proteins
During _______ the microtubules attach to kinetochore proteins at the centromere (where the sister chromatids are connected— middle) of the chromosomes
prophase
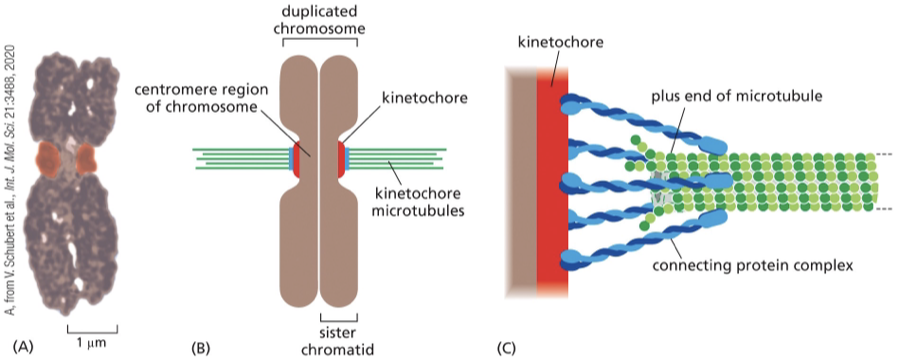
Each duplicated chromosome has ______ (#) kinetochores…
2 kinetochores one on each sister chromatid
Because these sister kinetochores face in opposite directions, they tend to attach to microtubules from opposite poles of the spindle; thus, each duplicated chromosome becomes linked to both spindle poles. The attachment to opposite poles, called bi-orientation, generates tension on the kinetochores, which are being pulled in opposite direction
What happens during anaphase?
sets of chromosomes are separate and are pulled towards opposite ends of the dividing cell
begins abruptly w/ breakage of cohesin linkages that hold the sister chromatids in the duplicated chromosome
How does APC/C trigger the separation of sister chromatids during anaphase?
before anaphase separase (protease) bound to securin (inhibitory protein) → seprase is inactive
Active APC/C catalyzes the ubiquitylation + destruction of securin→ securin is no longer bound and is freed
Securin is active!→ cleaves the cohesin complexes (allows the mitotic spindle to pull sister chromatids apart
ppt notes
•Once each sister chromatid has attached to the spindle APC (anaphase promoting complex) is activated
•APC ubiquitinylates resulting in the degradation of Securin (an inhibitory protein), causing the activation of Separase which degrades Cohesins to allow chromosome disjunction segregation
•APC also tags M cyclin for degradation resulting in the end of Mitosis
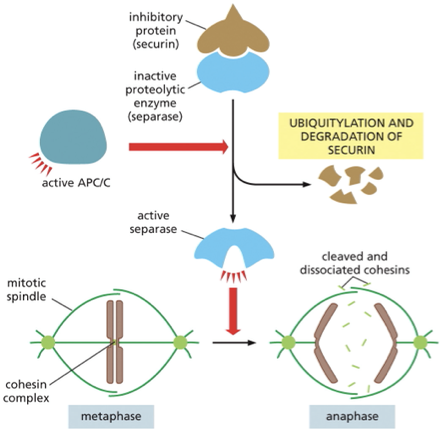
At the beginning of anaphase, securin is targeted for destruction by______
APC/C the same complex that marks M-cyclin for degradation
once securin has been destroyed separase is then free to break the cohesion linkage
By the end of anaphase?
the chromosomes have separated into two equal groups, one at each pole of the spindle
During telophase…
final stage of mitosis
mitotic spindle disassembles and the nuclear envelope reassembles around each cluster of chromosomes to form the 2 daughter nuclei
The nuclear envelope breaks down and re-forms during mitosis. The phosphorylation of nuclear pore proteins and lamins helps trigger the disassembly of the nuclear envelope at prometaphase. ______ of these proteins at telophase helps reverse the process.
Dephosphorylation
mitotic spindle is made primarily of ?
microtubules and microtubule-associated proteins, not actin
true or false
New microtubules grow out in random directions from the centrosomes.
true
The anaphase-promoting complex or cyclosome (APC/C) triggers the onset of anaphase by ?
triggering the cleavage of the cohesins that hold the sister chromatids together
cytokinesis
cell is cleaved into 2 — completes the M phase
contractile ring
structure made of actin and myosin filaments
forms a belt around a dividing cell → pinches it in 2
Apoptosis
tightly controlled form of programmed cell death
allows excess cells to be eliminated from an adult or developing organism
Part of the normal developmental plan
Also induced by infection, DNA damage, and lack of cell signaling
during development apoptosis can remove cells that are not needed
example: mouse paws + our own hands and feet started like weblike hands the individual fingers and toes separate because the cells between them die during embryonic development
example: tadpole→ tadpole changes into a frog→the cells in its tail die, and the tail, which is not needed in the adult frog, disappears
in adult tissues organ size is kept in check by ?
a balance between cell death and division
what is cell necrosis
cells that die as a result of acute injury
swell and burst
spew their contents across their neighbors
eruption→ inflammatory response
this is different than apoptosis which doesn’t damage its neighbors
A cell in the midst of apoptosis may develop irregular bulges called?
blebs
on the surface but then it shrinks and condenses
Explain why Apoptosis is different than necrosis:
Apoptosis is different from necrosis resulting from an acute injury
• apoptosis is an (energy-dependent) process that involves activation of a death program, and ends with the phagocytosis of the resulting cell fragments
• cells that die from an acute injury (necrosis) just swell and lyse, releasing their contents to the extracellular space and causing inflammation (wound)
Caspases
what are the 2 types of capases
Caspases: cysteine- aspartic acid proteases
a protease that when activated mediates the destruction of a cell by apoptosis
a family of more than a dozen proteases that act as the executors of apoptosis, contain nucleophilic cysteine in their active sites. They cleave after aspartic acid residues in target proteins
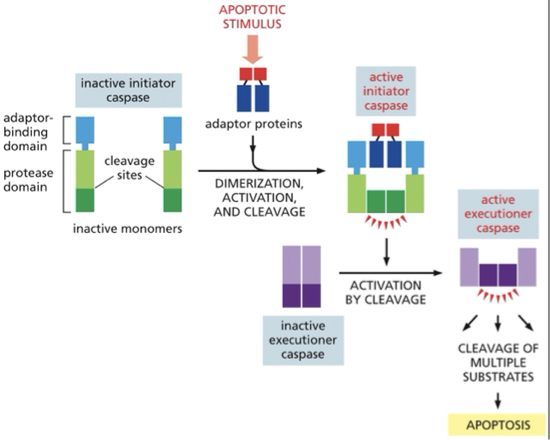
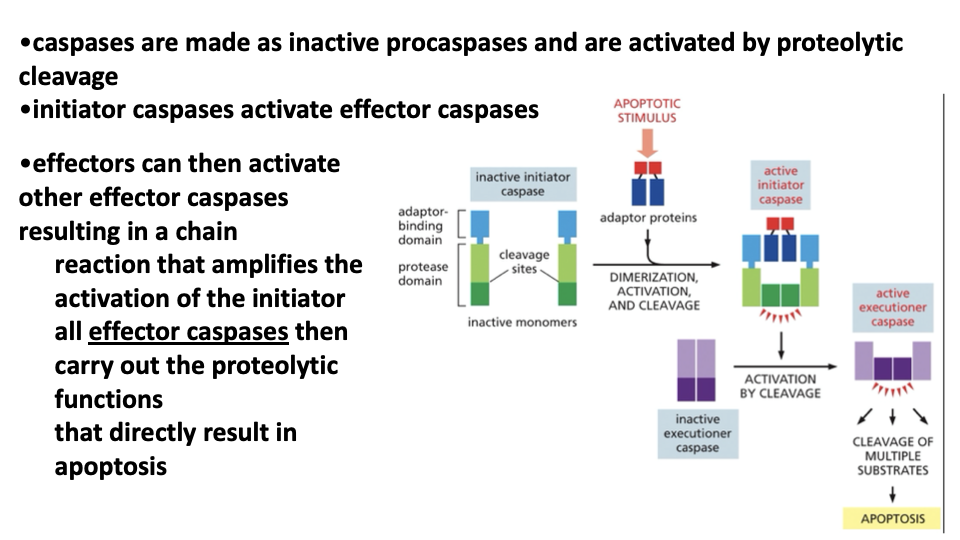
made as inactive precursors
activated in response to signals that induce apoptosis
What are the key targets if caspases
the inhibitor of a proapoptotic Dnase that digest chromosomal DNA
Cytoskeletal proteins
Nuclear lamins: there is an executioner caspace that targets the lamin proteins that form the nuclear lamina underlying the nuclear envelope. The cleavage causes a irreversible breakdown of the nuclear lamina.
What are the 2 types of caspases?
2 different types:
Initiator and executioner
initiator caspases→ cleave & activate downstream executioner caspases
initiator caspase is 1st made as an inactive monomer → apoptic signal triggers the assembly of adaptor proteins that bring together the pair of initiator caspases→ then activates executioner caspace by cleavage →leads to cleavage of multiple substrates → apoptosis
bcl2
suppresses apoptosis
was identified as an oncogene that contributes to the development of human B cell lymphomas
bcl-2 expression is activated in response to survival factors
Bcl-2 is an antiapoptotic protein: promotes tumor formation by inhibiting apoptosis
process:
survival factor binds to cell surface receptor→ activates intracellular signaling pathway→activates transcription regulator→ goes to nuclues→ activates gene encoding bcl2 (protein that inhibits apoptosis)
Bcl2 inhibits apoptosis by preventing _____ and _____ from releasing cytochrome C
Bax & Bak
bax and bak can trigger apoptosis by releasing cytochrome C, explain
apoptotic stimulus→ activated bax and bak molecules→ release cytochrome C from mitochondria → activates adaptor protein → creates apoptosome ( 7 armed complex) → recruits caspase 9 molecules → activates caspase 9→ leads to caspase cascade leading to apoptosis
Cytochrome C release from the mitochondria triggers activation of the
apoptosome
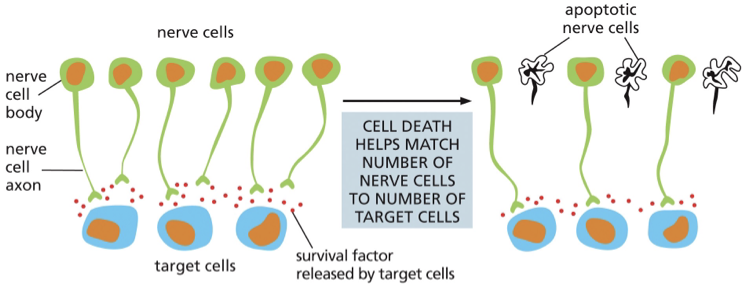
A large number of neurons are produced during development
•neurons depend on the _________ _______secreted by the target cells, but the amount of survival factor secreted is not sufficient to promote survival of all the original neurons
survival factors
more details:
•up to 50% of neurons die
•ensures that all target cells are contacted by nerve cells
cell death can help adjust the number of target cells by contact
Myostatin
signals to prevent growth of muscle cells
•Mutations lead to nearly un-controlled muscle growth

what you should know
•How does apoptosis differ from necrosis?
•Which signals or processes promote apoptosis and which inhibit apoptosis?
•How or why does mitochondrial damage lead to apoptosis?
•What molecules regulate the timing and sequence of cell cycle phases?
•What target proteins are regulated by M-Cdk?
•What target proteins are regulated by S-Cdk?
•How is M-Cdk activity negatively-regulated and how is it positively-regulated?
•Which proteins function as tumor suppressors in animal cells and how do they act?
•What controls the cell cycle in yeast cells?