CHEM 20A: Lectures 1 - 10 (Vocab and Experiments)
1/49
Earn XP
Description and Tags
Vocab definitions and experiments' diagrams and significance (based on lecture notes)
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
50 Terms
Extensive Property
a property that depends on the size of the substance
ex: volume, mass
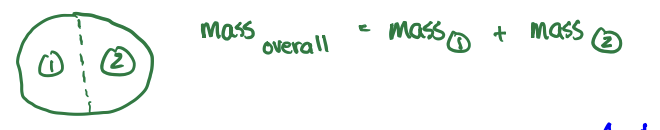
Intensive Property
a property that is independent of the size of the substance
ex: temp, density
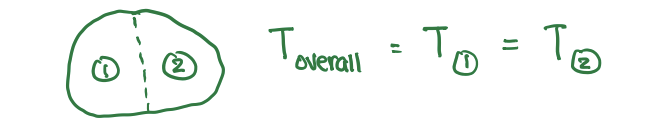
Law of Conservation of Mass (Lavoisier)
Experiment: decomposition of mercury oxide
Significance: not creating or destroying building blocks, but rearranging in chemical reactions; you combine them in different ways and it comes out with a different property
Law of Definite Proportion (Berthollet vs. Proust)
Experiment: Lavoisier’s experiment many times and measure [mass of gas/mass of mercury oxide]
Berthollet said % mass of gas is not fixed; it can vary over a range
Proust said, in a given chemical compound, the proportions by mass of elements that compose it are fixed; variations due to error or impurities
Significance: building blocks combine in specific ratios (ONE COMPOUND) to form different compounds
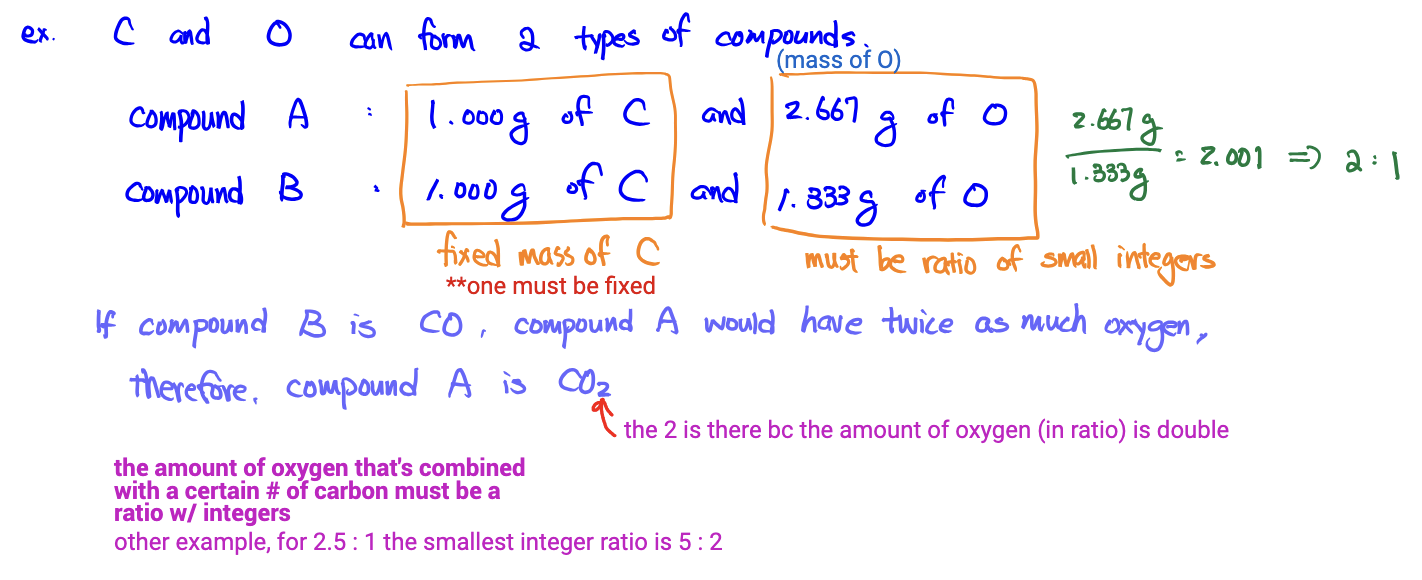
Law of Multiple Proportions
When two elements form a multiple compounds (DIFFERENT COMPOUNDS WITH SAME ELEMENTS), the masses of one element that combine with a fixed mass (1g) of the other element are in the ratio of small integers to each other.
*note: when solving, one element should be fixed
Dalton’s Atomic Theory of Matter (1808)
Significance: summarized the conservation of mass and definite proportion; coined “atom”
The two main things he got wrong:
He said that atoms are indestructible/indivisible
Can be further broken down to electrons, protons, and neutrons
He assumed the identity of the atom is determined by the mass
Not true because of isotopes
Law of Combining Volumes (Gay-Lussac)
The ratio of the volumes of any pair of gases in a gas phase chemical reaction (at the same temperature and pressure) is the ratio of simple integers.
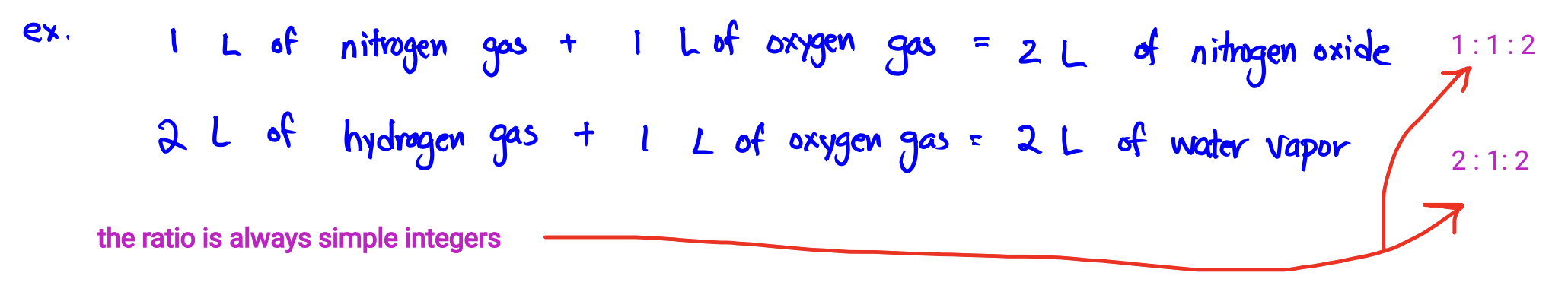
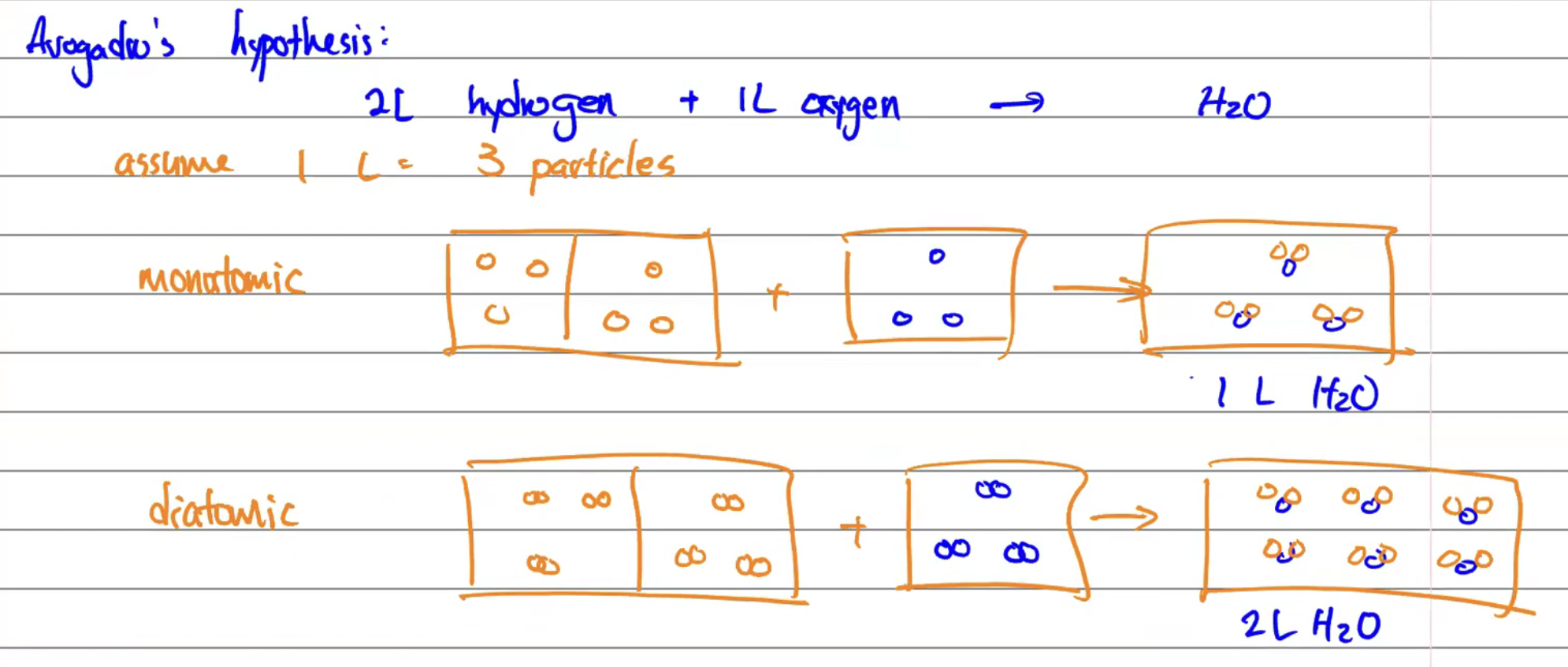
Avogadro’s Hypothesis
Equal volumes of different gases at the same temperature and pressure contain equal numbers of particles.
Experiment: 1 L of N + 1 L of O = 2 L of NO
The assumption would be that it would = 1 L NO, but since it’s 2 L NO, the hypothesis suggests N and O are diatomic molecules
Mole
Conceptualization: a unit for an amount of a substance, kinda like how a pair = 2 and a dozen = 12
Formula: 1 mol of atoms = 6.0221 × 10^23 atoms
Avogadro’s Number: 6.0221 × 10^23
If I divide mass (g) by the molar mass, I get mol of molecules
Avogadro’s Number
6.0221 × 10^23 atoms
Relationship between average atomic mass and mole
avg. atomic mass in amu = 1 mol of [element] atoms
![<p>avg. atomic mass in amu = 1 mol of [element] atoms</p>](https://knowt-user-attachments.s3.amazonaws.com/812f5fbf-ebae-437c-9f2f-3f477bdfd2ba.png)
Molar Mass of an Element
molar mass of an element = mass of one mole of its atoms (atomic mass)
Molecular Weight (molar mass of a compound)
molar mass of a compound = mass of 1 mole of its molecules
To find the molar mass of a compound, add up the avg. atomic mass of all the atoms in a molecule
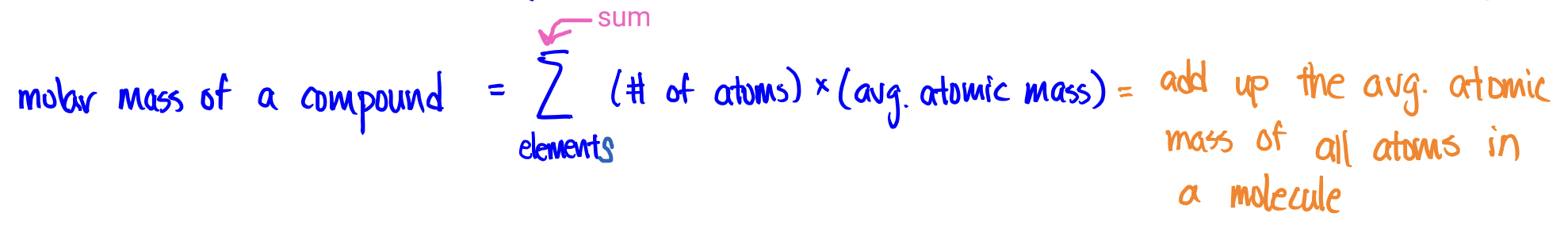
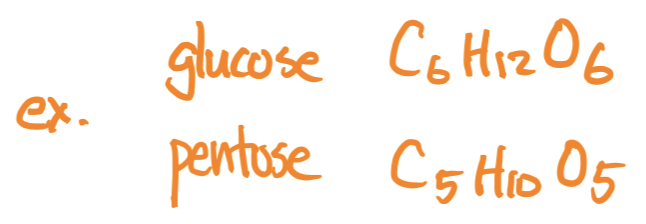
Molecular Formula
the actual number of atoms in a molecule
Empirical Formula
the simplest whole number ratio of atoms
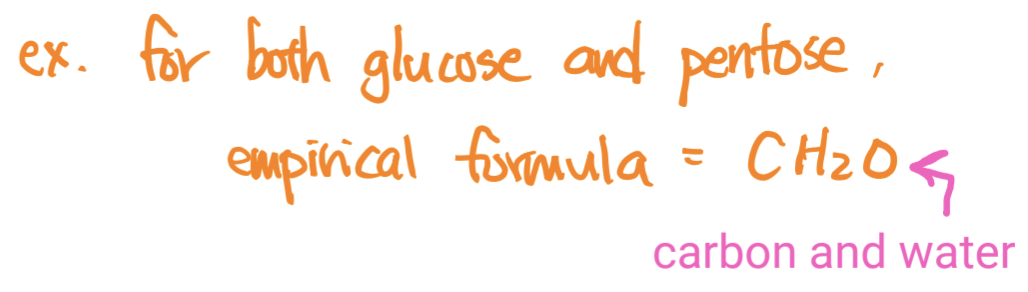
Percent Mass Composition
(mass of element in a compound)/(mass of a molecule)

Empirical Formula from Percent Mass Composition
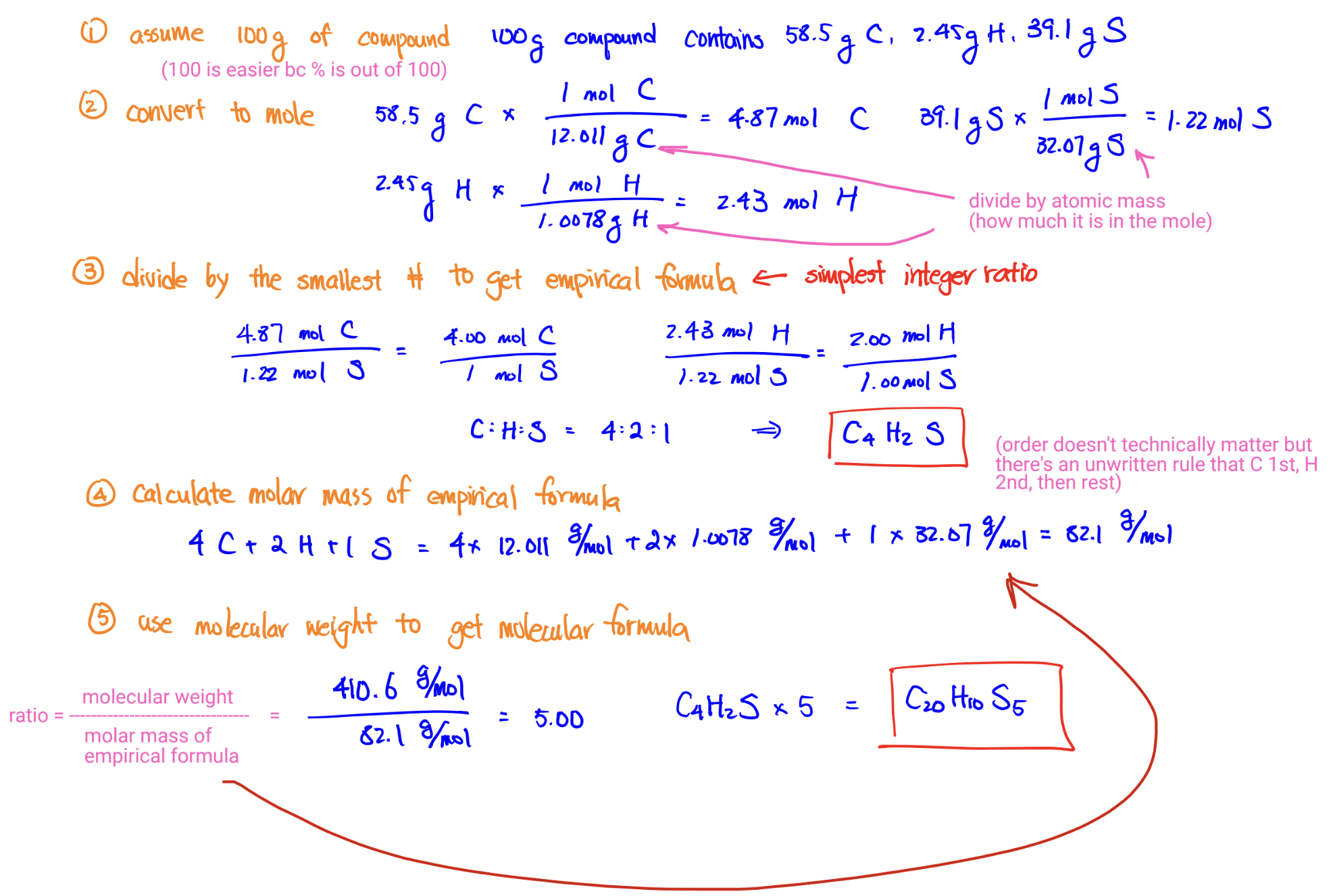
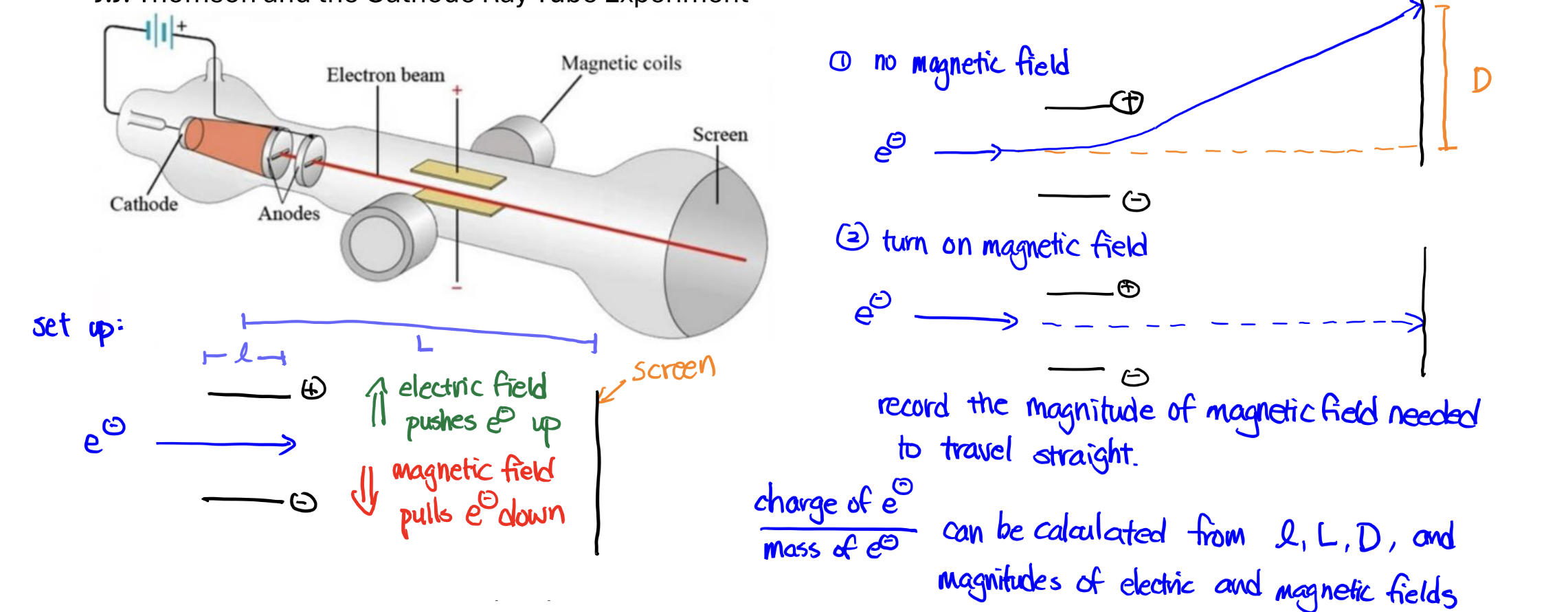
Cathode Ray Tube Experiment
Experiment: shoot a ray of electrons through a tube with an electric and magnetic field affecting the ray; electric directs the path, and changing the magnetic field can make it go straight (the light can curve without magnetic field, the distance = D)
Significance: charge-to-mass ratio
Oil Drop Experiment
Experiment: oil drop given a charge, and when it falls (gravity pulls it down), the negatively charged drop is suspended in air by changing the magnitude of the electric field
Significance: charge of e- (it’s 1.6 × 10^-19)
(charge can be calculated from the mass of the oil drop and the magnitude of the electric field needed to balance g-force)
If there are multiple e-s, the difference in charges has to be an integer multiple of the charge of the e-, and the smallest difference is the charge of the e-
Gold Foil Experiment
Before, the Plum-Pudding Model said an atom was made up of a positively charged “pudding” with e-s evenly spread out because they repel.
However, when a sheet of gold was shot with positively charged particles, there were some that were deflected/bounced back.
This suggested that atoms have a core (the nucleus) that’s dense and positively charged
Significance: discovery of a nucleus
Mass Spectrometry
A magnetic field bends the path of e-s in a beam, and it was seen that some atoms of the same element were bent differently. Lighter atoms bend more, and heavier atoms bend less.
Significance: atoms of the same element CAN have different masses; discovery of isotopes
Calculation of Average Atomic Mass
SUM of all isotopes’ [(mass x abundance)]
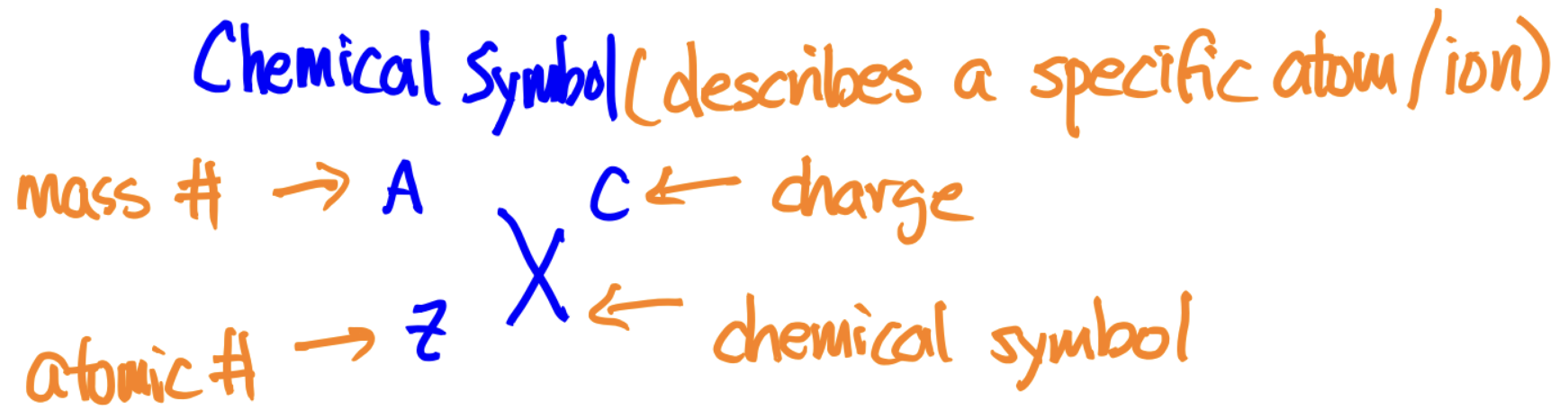
The mass of each is denoted by the superscript before the chemical symbol (the superscript after is the charge)
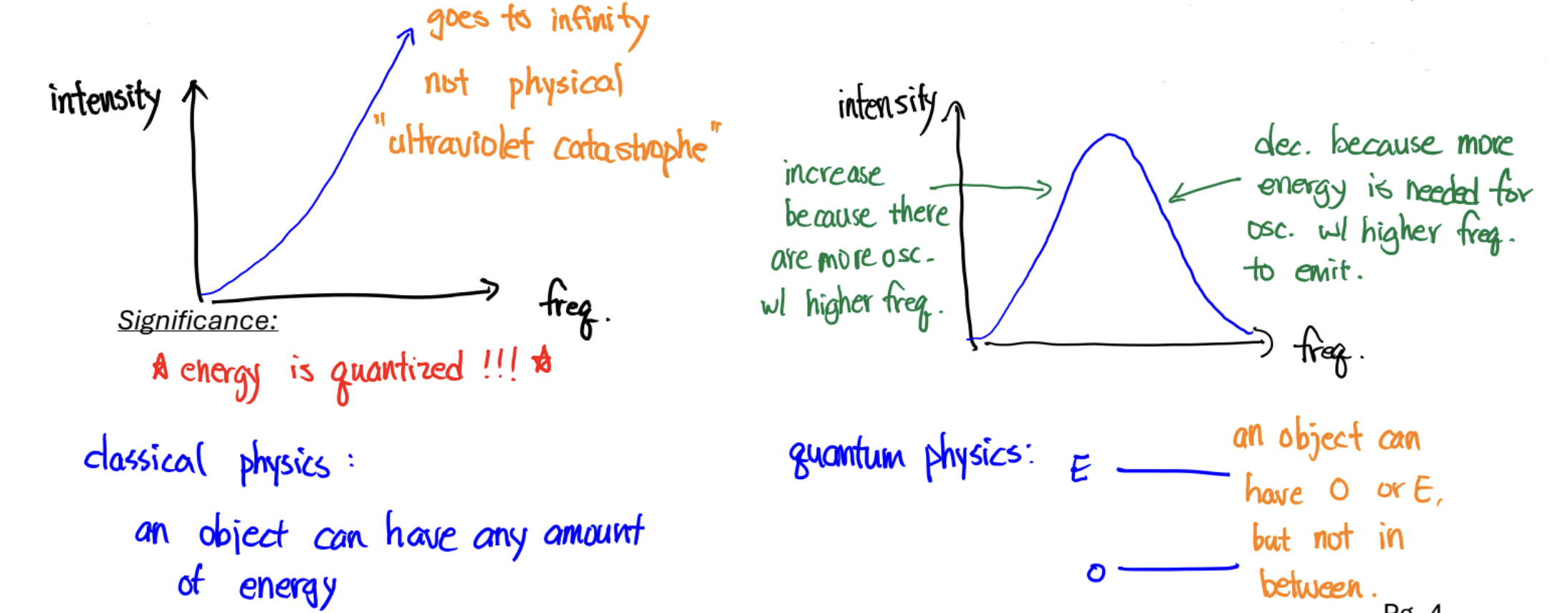
Blackbody Radiation
Significance: energy is quantized
For a blackbody (object), as temp increases, the frequency of its most intense color increases. This is because more energy is needed to turn on the light bulbs of higher frequency (energy to turn on/# of light bulbs is proportional to freq).There is more energy as temperature increases, so the higher frequency bulbs can be turned on. The “lighting up” of the higher frequency bulbs means the most intense color also shifts to a higher frequency.
Classical Physics vs. Planck (and quantum physics)
Classical Physics:
energy shared equally to all light bulbs
# of light bulbs proportional to its frequency
it can go up to infinity/objects can have any amount of energy
HOWEVER: not physical (doesn’t actually happen); “ultraviolet catastrophe“
LBs must have energy to give off electromagnetic radiation & # of LBs proportional to freq…
Planck:
energy is not distributed equally
energy given to each light bulb is proportional to the frequency
more energy needed to give energy to LB w/ high freq
fewer high freq LB can emit
ENERGY QUANTIZED!!!
quantum physics: object can have E or O but no b/w
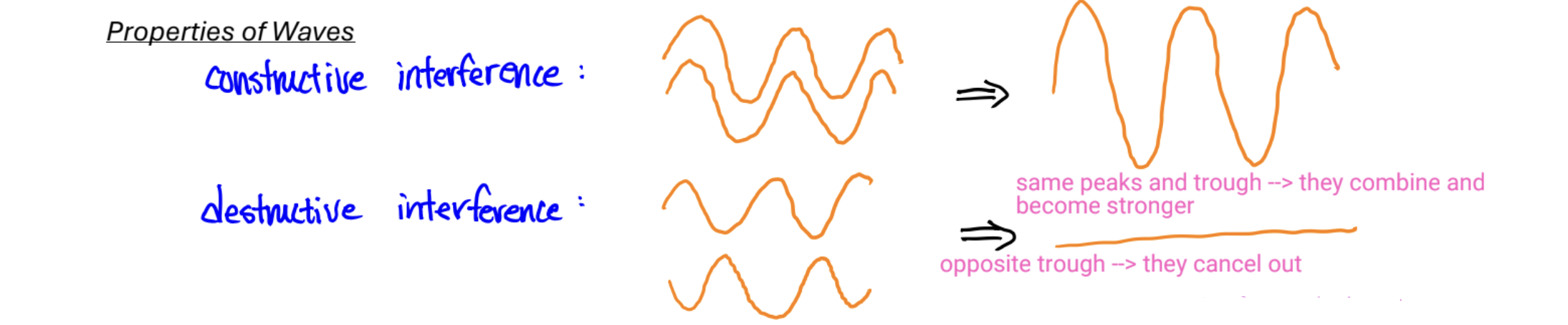
Properties of Waves
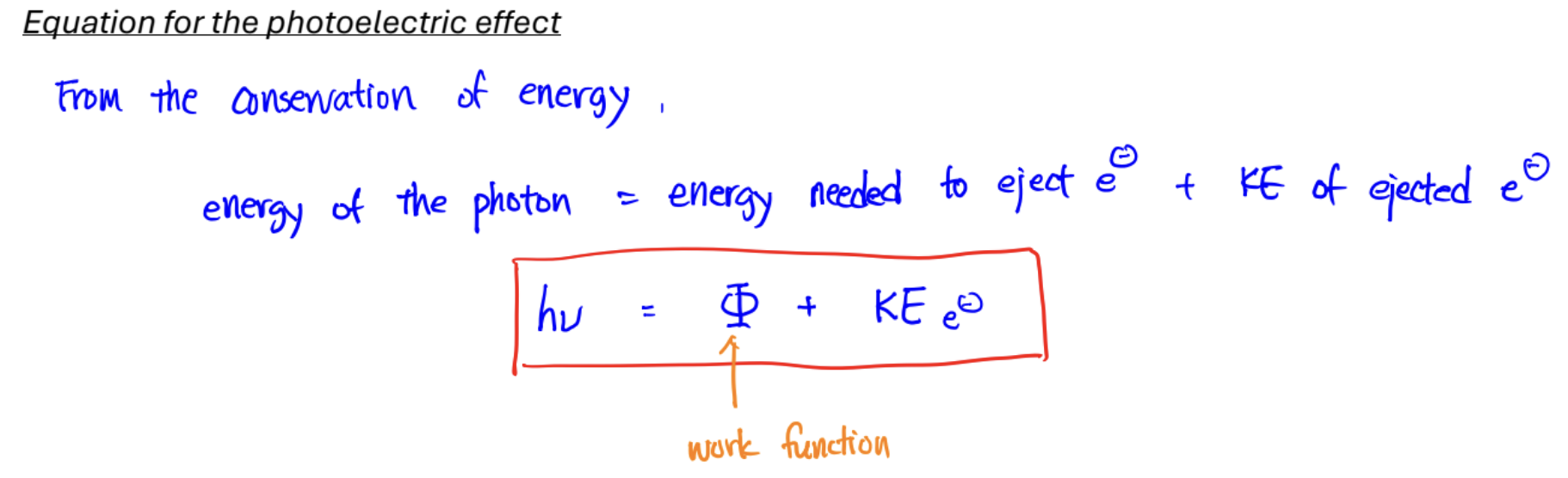
Photoelectric Effect
Experiment: shine light onto a metal plate; electrons bounce off
Einstein’s Explanation:
light exists as packets of energy, aka photons
the energy of a photon proportional to its freq: E = h*ν (ν is freq)
each photon can eject an e- if it has enough energy to break bond b/w metal and e-
leftover energy becomes KE of e- (@ threshold freq, it has just enough energy to break, KE = 0)
Significance: discovery of photons
Experimental observations:
higher intensity of light causes → more photons per sec → more e- ejected, but the KE of the ejected e- stays the same because energy of photon (E = h*freq) stays the same
There is a threshold frequency for different metals. Below the threshold frequency, photons don’t have enough energy to ejected e-
Increasing the frequency of the light → photons have more energy → increases KE of the ejected e-
de Broglie wavelength (to find wavelength for PARTICLE, not photon)
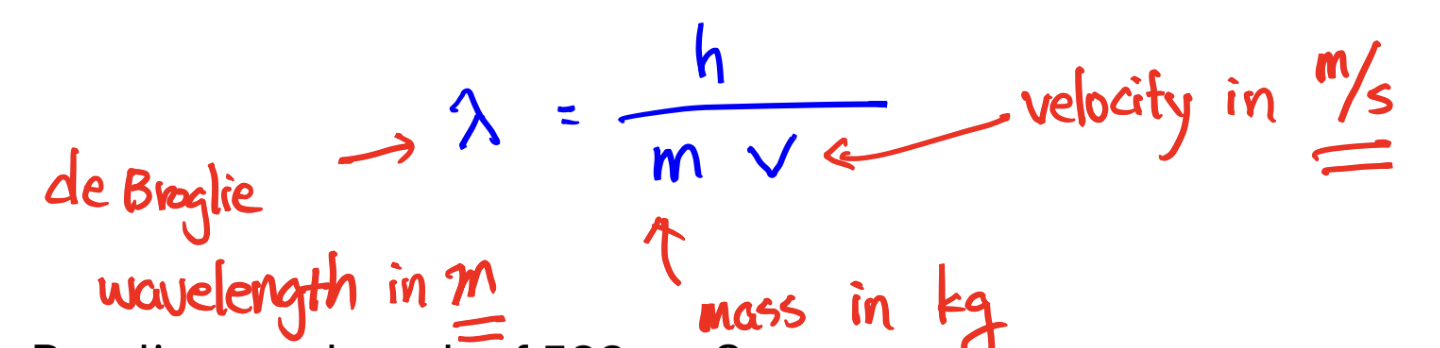
Two-Slit Experiment
Experiment: light shone on a screen with two slits.
if e- behaves like particle, no interference and light goes straight through to create two solid lines corresponding to the slits
BUT multiple lines were created
This suggested that, instead of behaving like particles, e- behaves like waves and that resulted in the interference pattern (distance b/w bright spots related to wavelength)
bright spot: constructive interference
dark spot: destructive interference
Significance: e- has wave-like behavior
Composition of Atoms
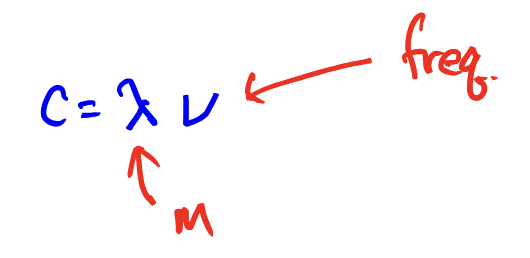
Electromagnetic Radiation: equation for calculating the wavelength of a PHOTON from its frequency
Electromagnetic Radiation: calculating the energy of a PHOTON from its frequency or wavelength
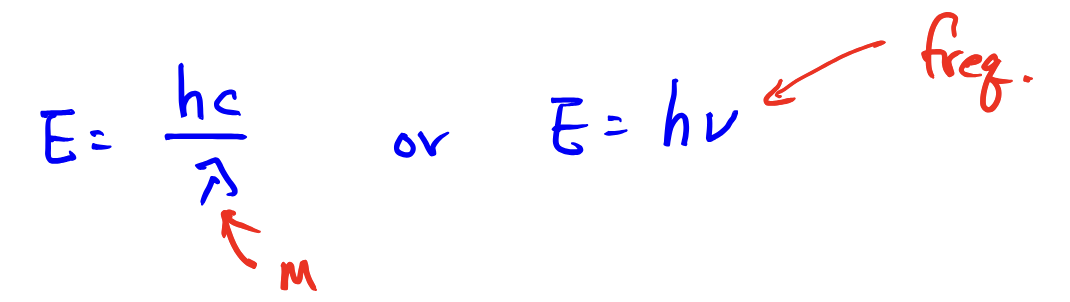
Absorption and Emission of Photons: definitions and equation
Absorption: when an e- absorbs a photon, it will jump to a higher level (only if the energy of the photon matches the energy difference)
Emission: when an e- drops to a lower level, it releases a photon whose energy is the differenc
Equation: for both,
E photon = |delta E| = |E of final - E of initial|
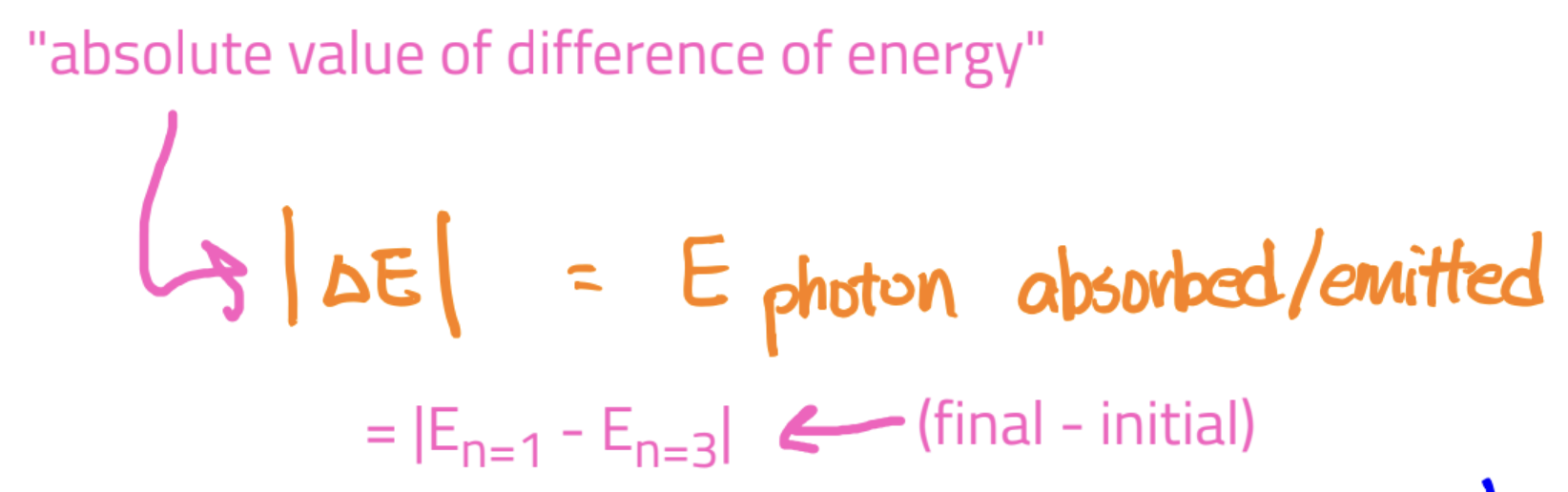
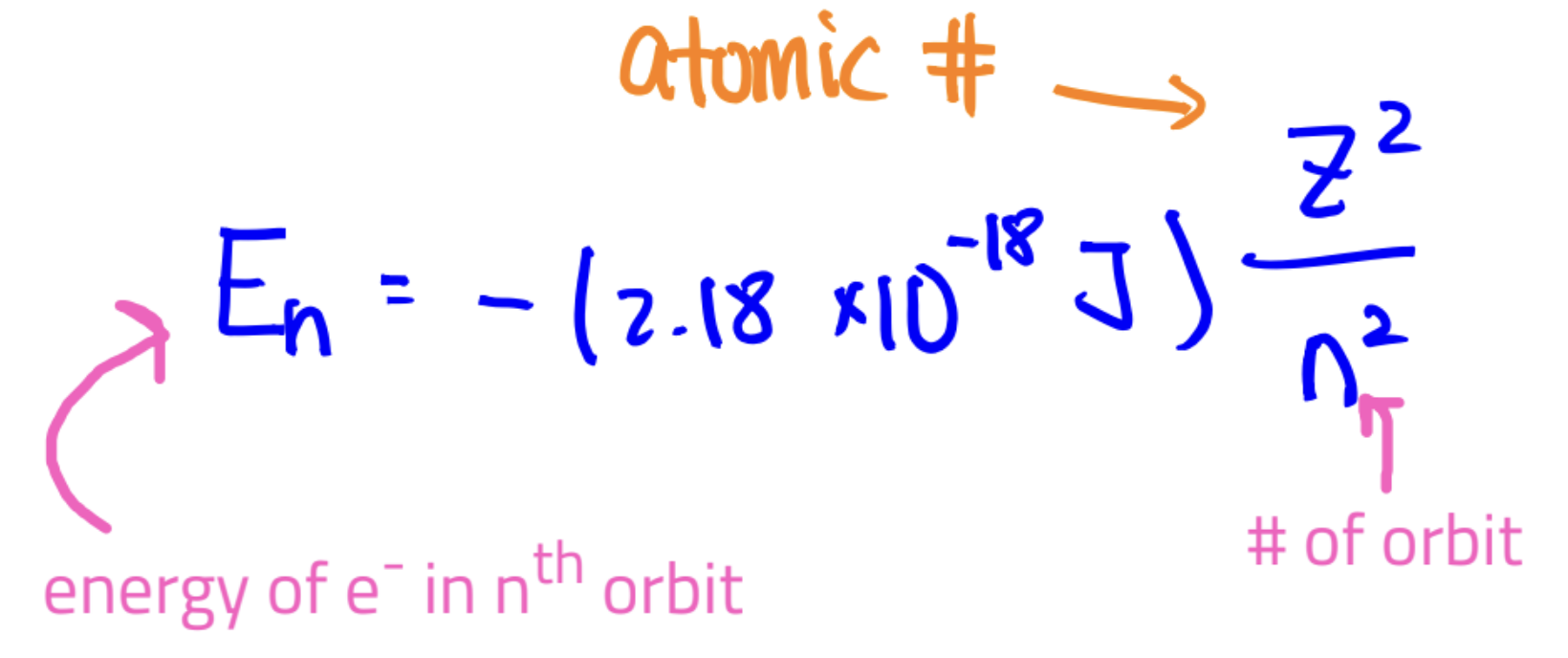
En for Bohr atom question
En for particle-in-a-box question
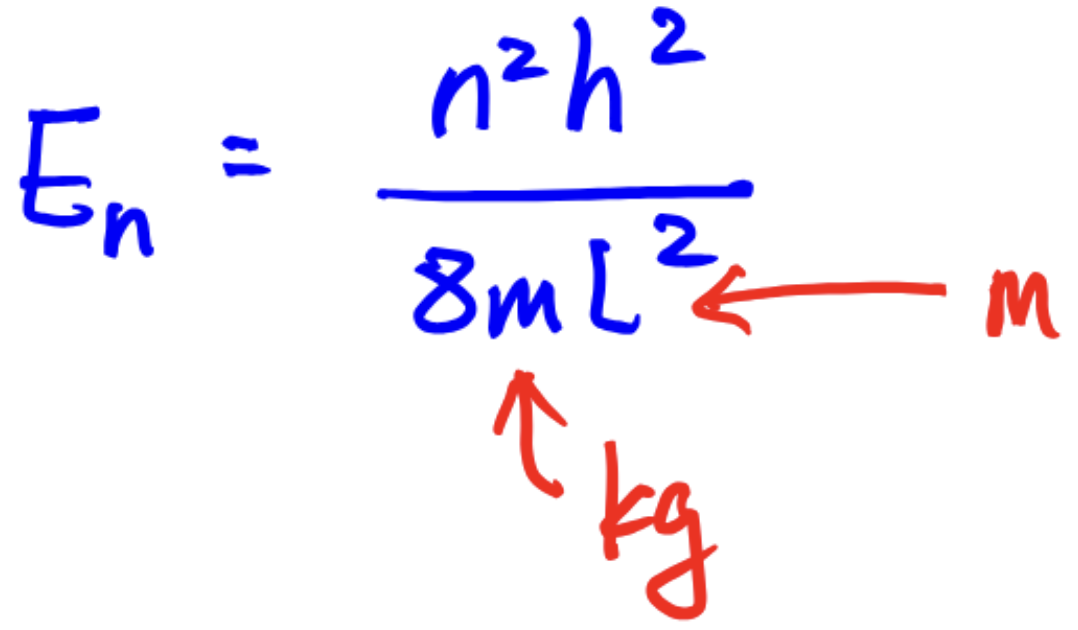
Wavefunction
a mathematical equation that describes the amplitude of a wave at different locations;
it has no physical meaning, but (wavefunction)² gives the probability of finding e-
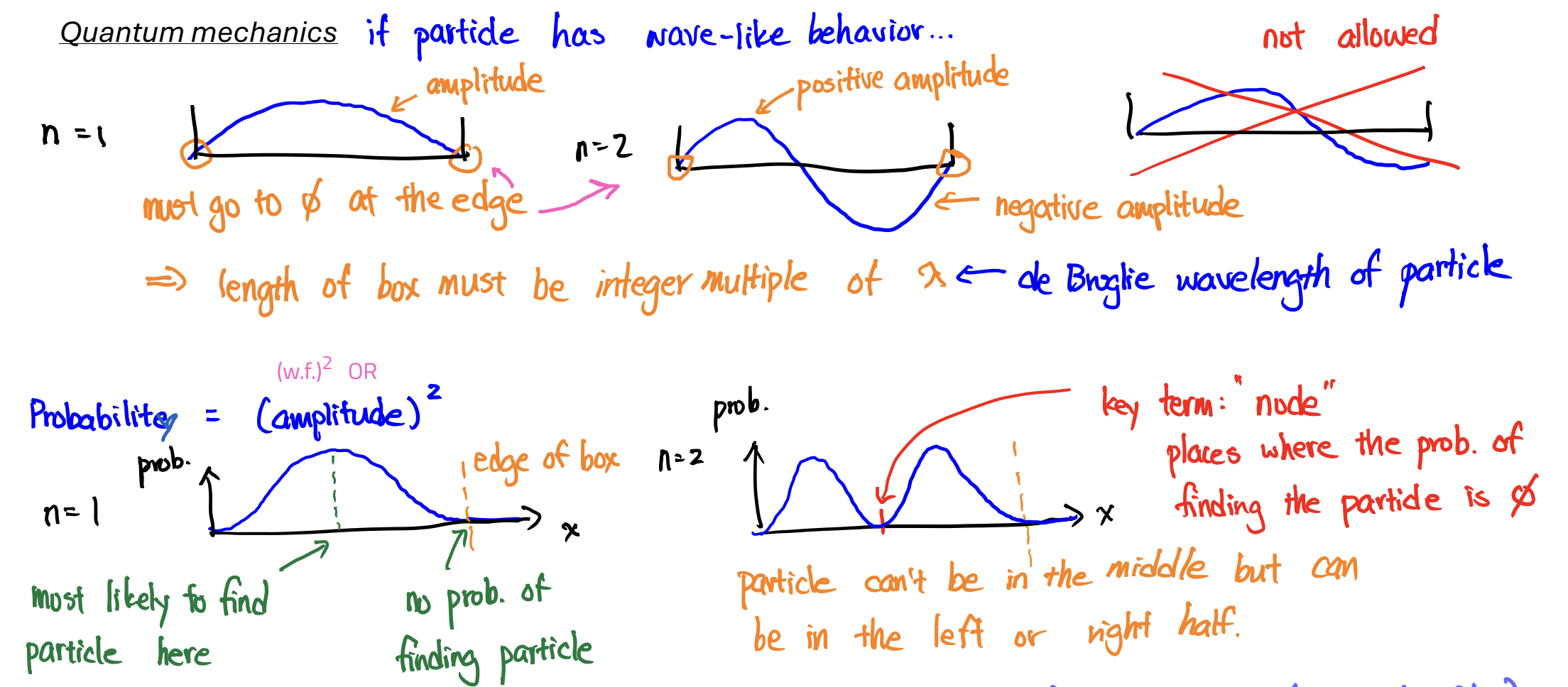
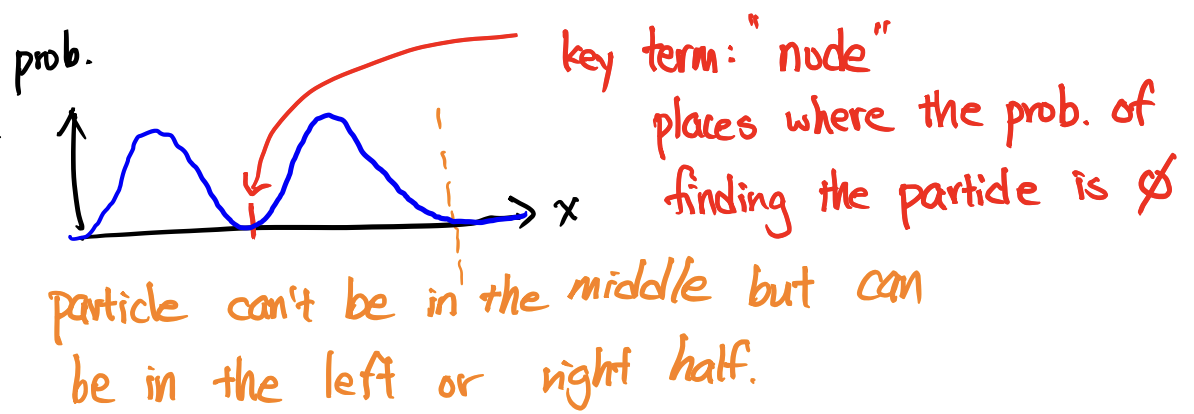
Node
probability of finding the particle is 0
n (shell)
Name: Principal quantum number
Value: 1, 2, 3,…
Determines: size
Rules: smallest allowed value is 1
As 𝑛 increases, the probability of finding the electron farther away from the nucleus increases.
L (subshell)
Name: Angular momentum quantum number
Value: n - 1
Determines: shape
Rules: smallest allowed value is 1; largest, n-1
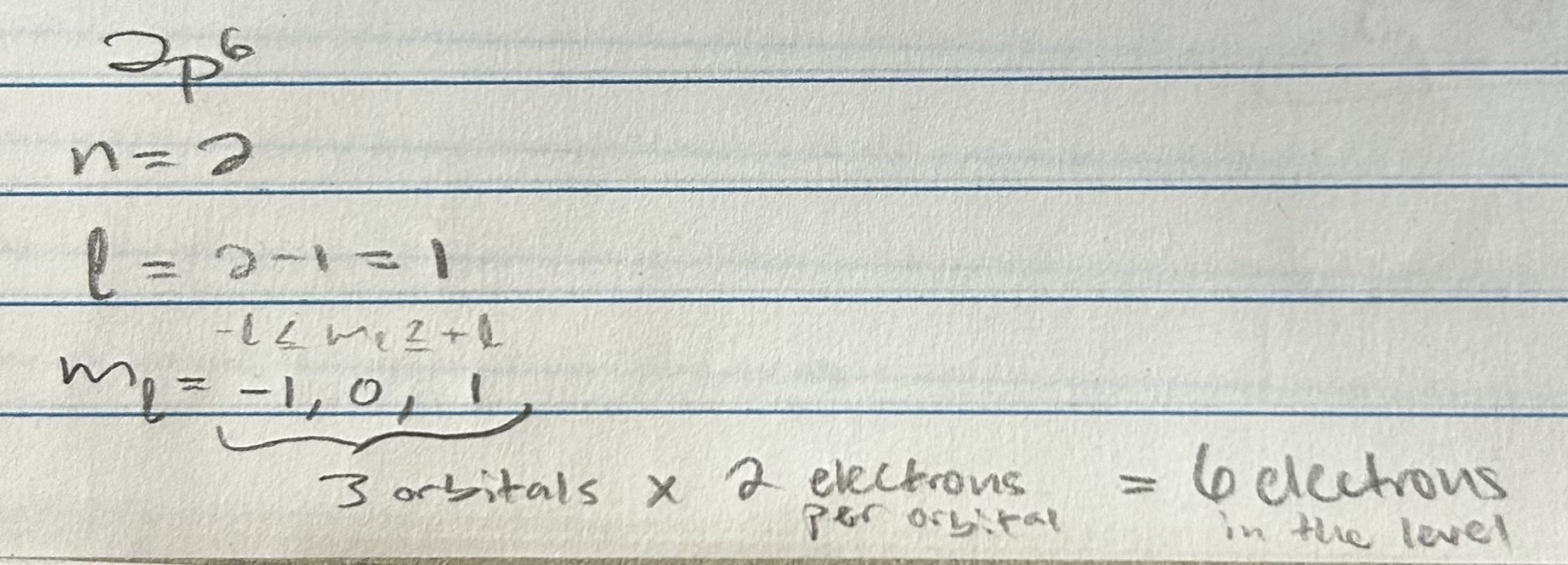
mL (orbital)
Name: Magnetic quantum number
Value: -L ≤ ml ≤ +L (the end points and what falls in the range)
Determines: orientation
Rules: allowed values depend on L
mS (spin)
Name: Spin quantum number
Value: +1/2, -1/2
Determines: spin of e-
How many subshells and orbitals are in the n = 3 shell?
(note: doesn’t specify L)
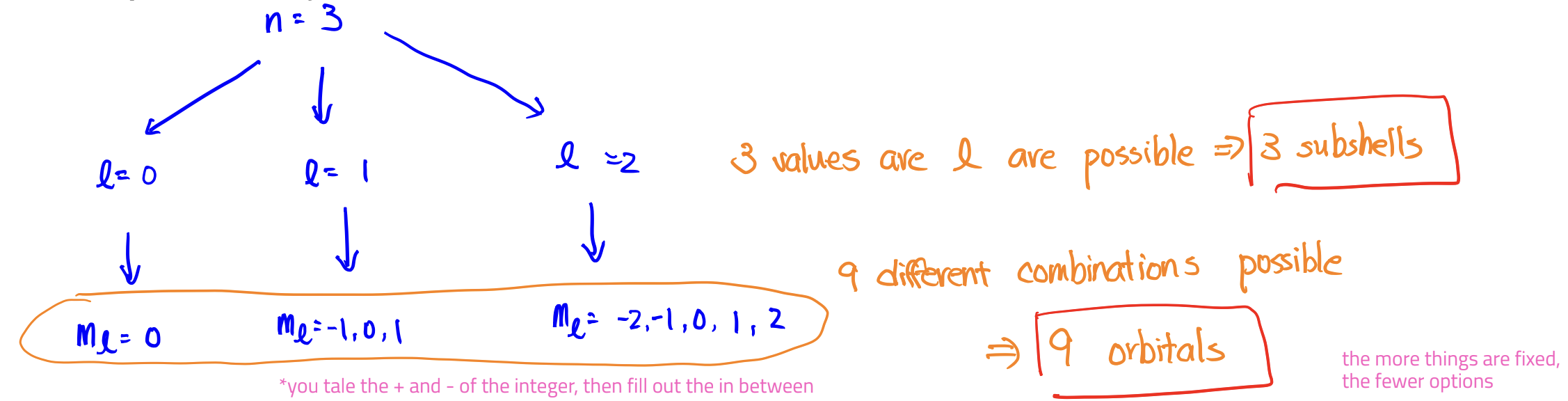
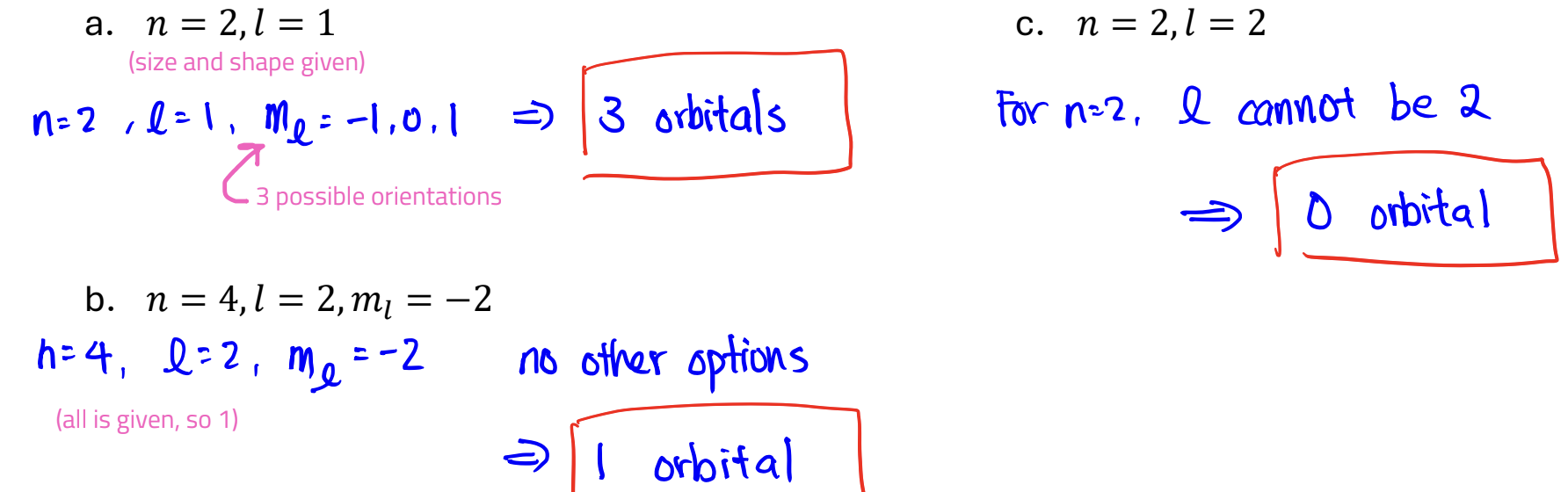
How many orbitals can have the following quantum numbers in an atom?
a. n = 2, l = 1
b. n = 4,l = 2, ml = −2
c. n = 2,l = 2
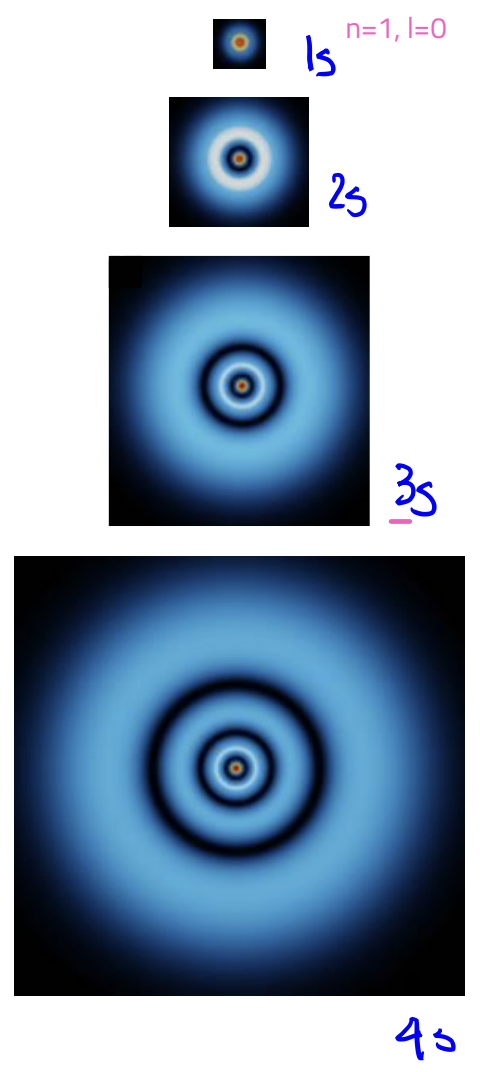
s-orbital
L = 0
angular nodes: 0
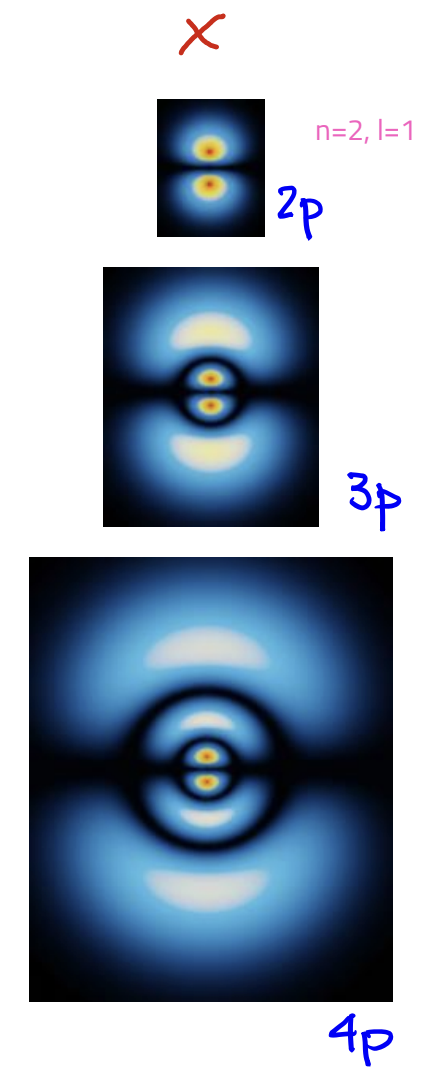
p-orbital
L = 1
angular nodes: 1
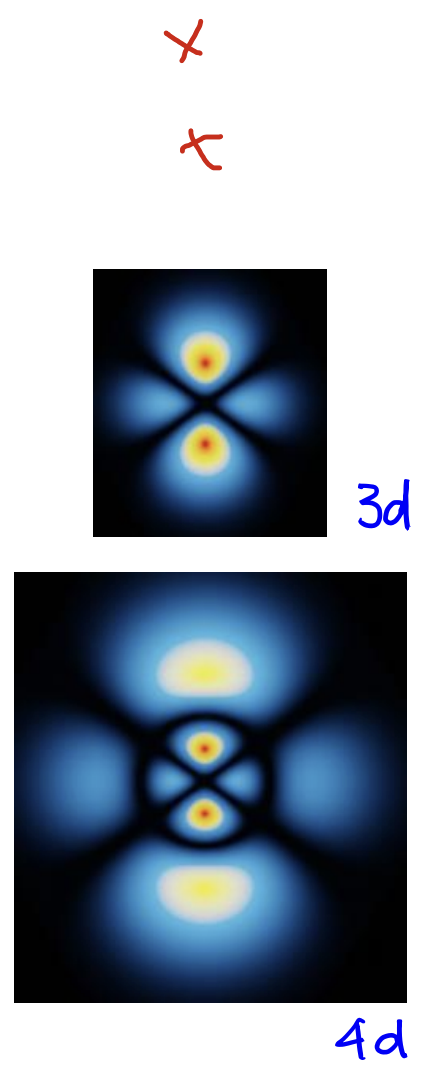
d-orbital
L = 2
angular nodes: 2
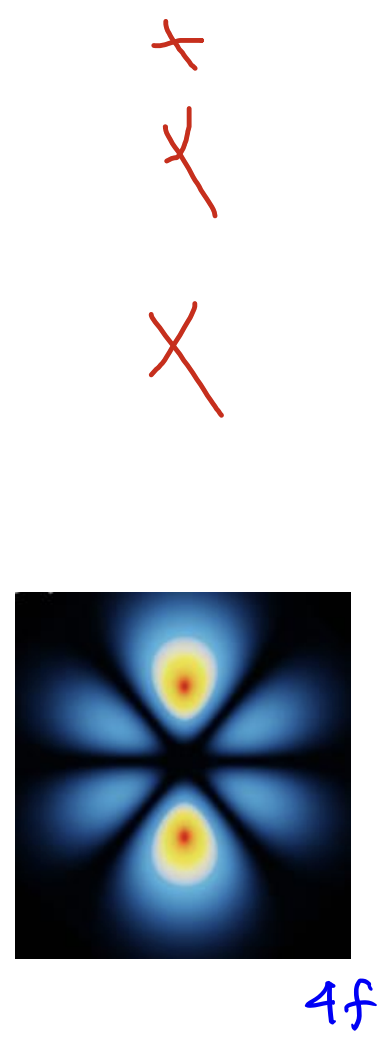
f-orbital
L = 3
angular nodes: 3
Effective Nuclear Charge
When there are multiple e-, e- found closer to the nucleus are less shielded
(if it’s closer, there are less other e- blocking; e-e repulsion is what causes the sheilding)
They will have a higher effective nuclear charge…
(it will feel more nuclear charge since it’s closer),
…making its energy lower (the stronger the attraction b/w the e- and nuc., the more stable and lower energy)
Energy Levels: Bohr vs Atomic Orbitals
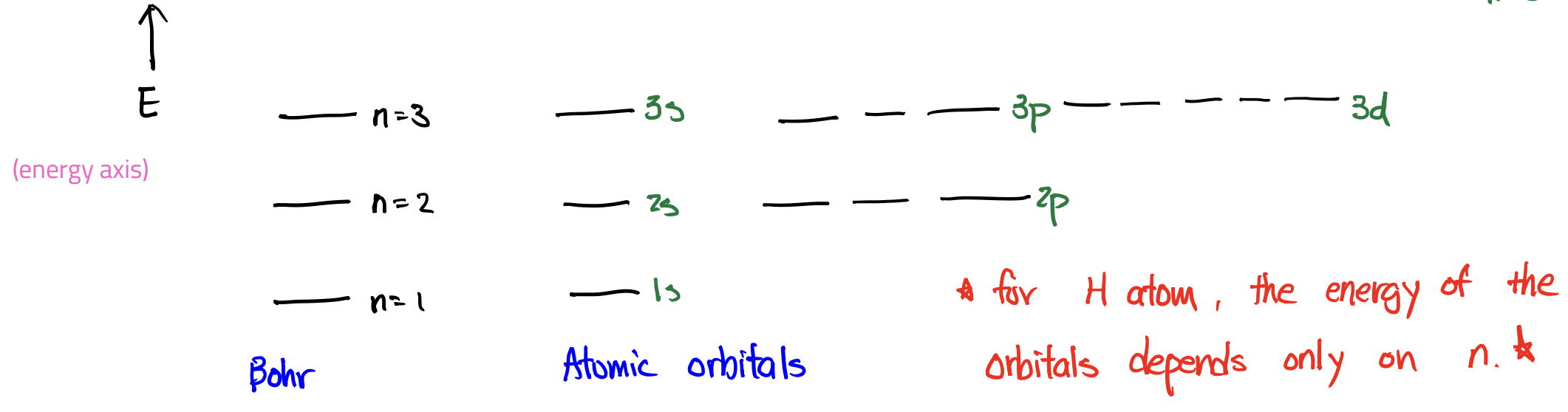
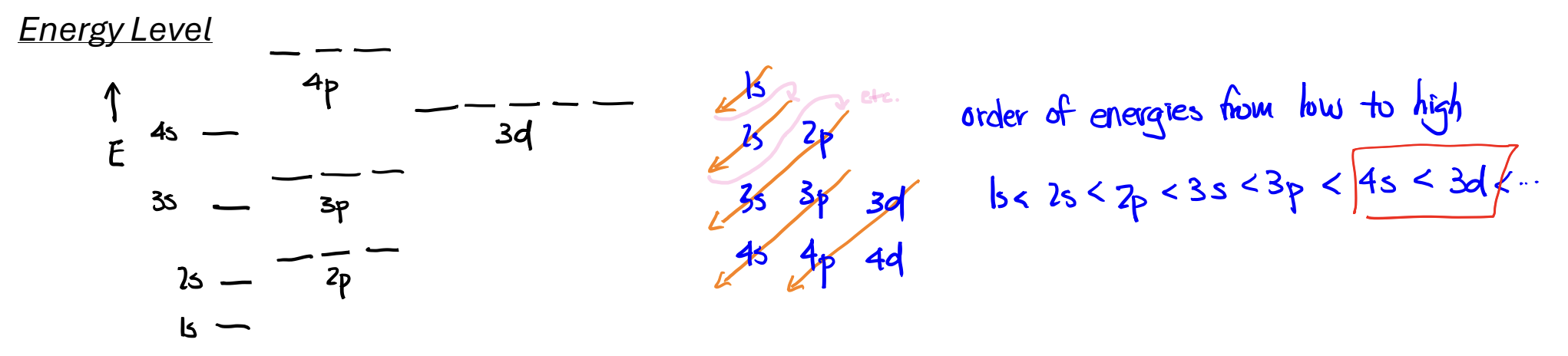
Energy Levels: Atomic Orbitals
General Rules for Writing Electron Configuration
Aufbau Principle: add Z electrons, one after another, to the orbitals with the lowest energy,
Pauli’s Exclusion: no more than two electrons in any one orbital.
Hund’s Rule: If more than one orbital in a subshell is available, add electrons with parallel spins to different orbitals of the subshell rather than pairing two electrons in one of the orbitals (if all full w/ 1, then ok to add a 2nd)